Mantidactylus steinfartzi, Scherz & Crottini & Hutter & Hildenbrand & Andreone & Fulgence & Köhler & Ndriantsoa & Ohler & Preick & Rakotoarison & Rancilhac & Raselimanana & Riemann & Rödel & Rosa & Streicher & Vieites & Köhler & Hofreiter & Glaw & Vences, 2022
|
publication ID |
https://doi.org/ 10.11646/megataxa.7.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:2FD8C310-6486-4592-92F6-5EB894EBD6AC |
|
DOI |
https://doi.org/10.5281/zenodo.7504367 |
|
persistent identifier |
https://treatment.plazi.org/id/BD10DEFC-468F-490B-A0F1-89B56128A6B1 |
|
taxon LSID |
lsid:zoobank.org:act:BD10DEFC-468F-490B-A0F1-89B56128A6B1 |
|
treatment provided by |
Plazi |
|
scientific name |
Mantidactylus steinfartzi |
| status |
sp. nov. |
Mantidactylus steinfartzi sp. nov.
Identity and justification.—This lineage was considered as M. sp. 33 by Vieites et al. (2009) and M. sp. Ca33 by Perl et al. (2014) and Vences et al. (2018). It was depicted as ‘ Mantidactylus sp. aff. biporus “Tsaratanàna Antsahamanara”’ by Glaw and Vences (2007). It occurs in sympatry(although not strict syntopy)with its sister species M. schulzi but is distinguished from it by advertisement calls, morphology, and concordant differentiation of 16S and Rag-1. It was previously thought (e.g. Glaw & Vences 2007) to be related to M. biporus , but our phylogenomic tree firmly places it in the M. ulcerosus clade.
Holotype.— ZSM 658/2001 ( FGMV 2001.107 ), adult male (seen calling), collected by F. Andreone, F. Mattioli, J.E. Randrianirina, and M. Vences between 4–9 February 2001 at Antsahamanara ‘Camp 1’ (14.0450°S, 048.7844°E, ca 1000 m a.s.l.), Manarikoba forest , Tsaratanàna Massif, Diana Region, Madagascar. GoogleMaps A 16S barcode sequence of the holotype was obtained in this study and was included in the analysis.
Paratypes.—A total of 11 paratypes: ZSM 659/2001 ( FG / MV 2001.110 )and ZSM 663/2001 ( FG / MV 2001.118 ), two adult males, and ZSM 655/2001 ( FG / MV 2001.68 ), ZSM 657/2001 ( FG / MV 2001.98 ), ZSM 660/2001 ( FG / MV 2001.113 ), ZSM 661/2001 ( FG / MV 2001.116 ), four adult females, with the same collection data as the holotype; GoogleMaps ZSM 843/2003 ( FG / MV 2002.0810 ), and ZMA 19567 ( FG / MV 2002.2315 ), two adult males, and ZSM 844/2003 ( FG / MV 2002.0811 ) and ZMA 19568 ( FG / MV 2002.2317 ), two adult females, collected by F. Glaw, R.D. Randrianiaina, and M. Vences on 3 February 2003 at ‘Camp 1’ on the Manongarivo Massif (13.9770°S, 048.4220°E, 751 m a.s.l.); GoogleMaps UADBA-A uncatalogued ( FGZC 3791 ), specimen of unknown age and sex, collected by F. Glaw, O. Hawlitschek, T. Rajoafiarison, A. Rakotoarison, F.M. Ratsoavina, and A. Razafimanantsoa on 3 December 2012 near Ambodimandresy (13.7133°S, 049.4911°E, 778 m a.s.l.) GoogleMaps .
Diagnosis.— Mantidactylus steinfartzi sp. nov. is a member of the M. ulcerosus clade as revealed by the phylogenomic analysis, and sister to the sympatric M. schulzi . See Table 4 View TABLE 4 for a list of diagnostic morphological characters. The combination of a small body size of up to 28 mm, smooth to slightly tubercular dorsal skin, absence of dorsolateral ridges, large tympanum size in males (15–17% of SVL), presence of white spots on flanks, and absence or weak expression of a white marking on snout tip, distinguishes M. steinfartzi sp. nov. from most other species of Brygoomantis from other clades: members of the M. betsileanus clade typically have a distinct white marking on the snout tip and no white spots on the flanks, and (except for M. betsileanus and M. riparius sp. nov. which differ in the number of pulses per note; Table 4 View TABLE 4 ) a lower pulse rate in advertisement calls; members of the M. fergusoni clade are larger, have a more granular dorsal skin, no white spots on the flanks, and lower pulse rate in advertisement calls; and members of the M. biporus and M. inaudax clades have, as far as known, fewer pulses per note in advertisement calls ( Table 4 View TABLE 4 ). Within the M. ulcerosus clade, the new species differs by a distinctly smaller body size and several other characters from M. bellyi and M. ulcerosus . It is morphologically rather similar to its sister species M. schulzi which, however, usually has no white dots on the flanks and a more distinctly expressed white marking on the snout, more granular dorsal skin, a smaller tympanum in males, a slightly larger male body size, and also differs in details of advertisement calls: a rather irregularly emitted note of quite variable number of pulses in M. schulzi vs less variability in pulse number and emission of short series of usually two calls in M. steinfartzi sp. nov. For detailed distinction from new species described herein, see the respective species accounts. A full list of molecular diagnostic sites in the 16S gene of M. steinfartzi sp. nov. in pairwise comparisons to all other Brygoomantis species is provided as Supplementary appendix.
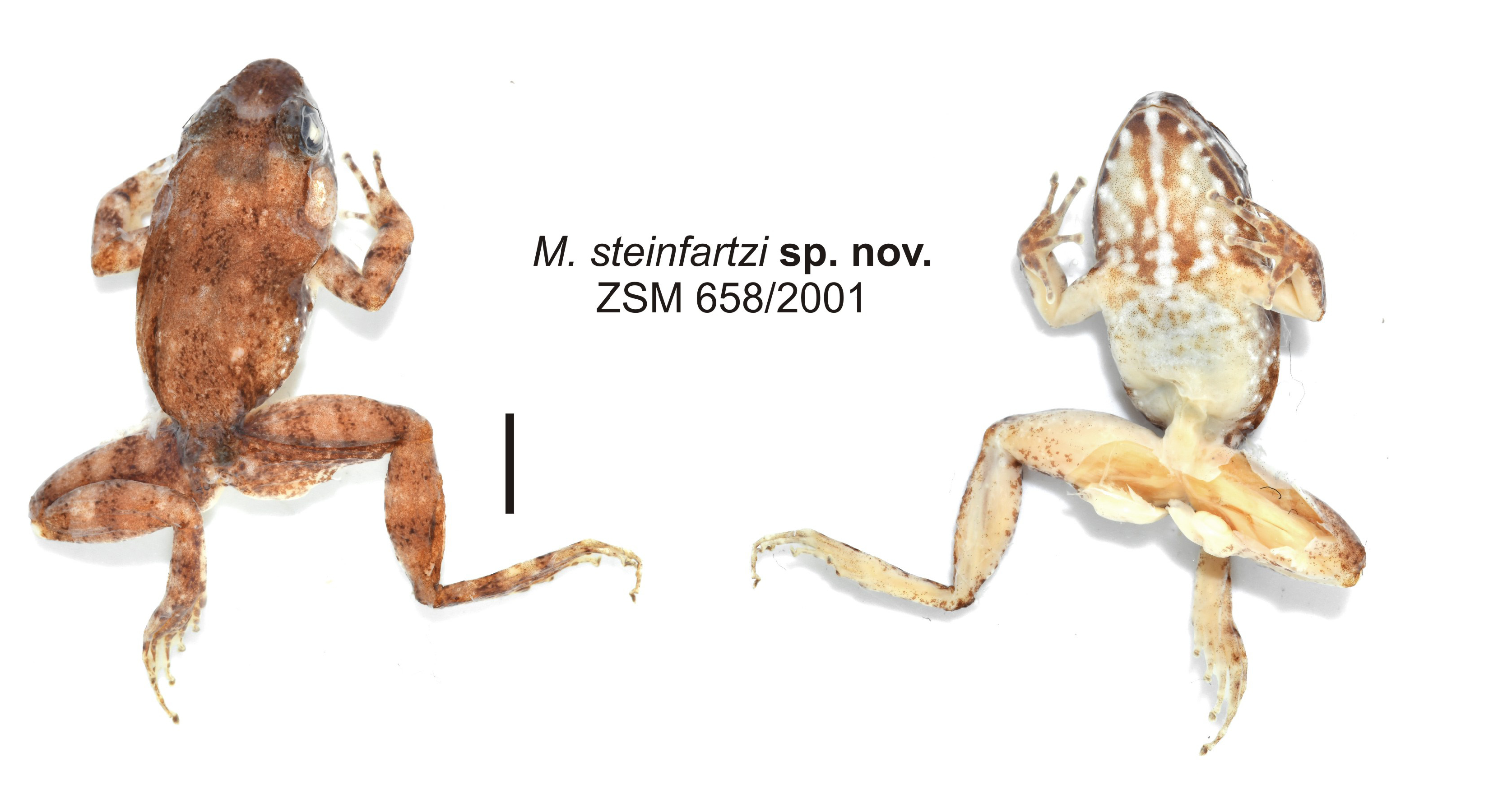
Description of the holotype.—Adult male in a mediocre state of preservation (softly fixed, similar to all other available specimens of this species; Fig. 23 View FIGURE 23 ); muscle tissue from left thigh removed, femoral glands partly detached for examination in internal view. Body rather stout. Head as wide as body. Snout rather pointed. Nostrils directed laterally, slightly protuberant, nearer to tip of snout than to eye. Canthus rostralis weakly recognisable, slightly concave; loreal region slightly concave. Tympanum distinct, large, wider than high, horizontal diameter of tympanum 106% of horizontal eye diameter. Supratympanic fold distinct, beginning straight above, with a rather distinct 45° bend midway towards insertion of forelimb, following the outline of the large tympanum. Tongue ovoid, distinctly bifid posteriorly. Vomerine teeth form two rounded aggregations, positioned posterolateral to choanae. Choanae rounded. Subarticular tubercles single. Inner and outer metacarpal tubercles present. Fingers without webbing. Relative length of fingers: I<II<IV<III. Finger discs slightly enlarged. Nuptial pads absent. Foot slightly shorter than tibia (97%). Lateral metatarsalia separated. Inner metatarsal tubercle present. Outer metatarsal tubercle very small but recognisable. Webbing formula: 1(0.5), 2i(1), 2e(0.5), 3i(2), 3e(1), 4i(2), 4e(2), 5(0.5). Relative length of toes: I<II<V<III<IV. Skin on the upper surface smooth (in life slightly granular), slightly glandular dorsolaterally. No dorsolateral ridges or folds. Ventral side smooth. Femoral glands large and very distinct in external view.
Colour in preservative: dorsally almost uniformly brown, with a few indistinct and irregular large markings. A somewhat darker patch is present between the eyes. Limbs with poorly contrasted dark crossbands. Flanks and sides of head with scattered whitish spots. Snout tip with a poorly contrasted light spot. Venter beige, throat and chest with brown pigment and a light medial line on the throat. Lower lip ventrally with distinct alternating light and brown spots. Colouration in life not recorded for holotype specimen.
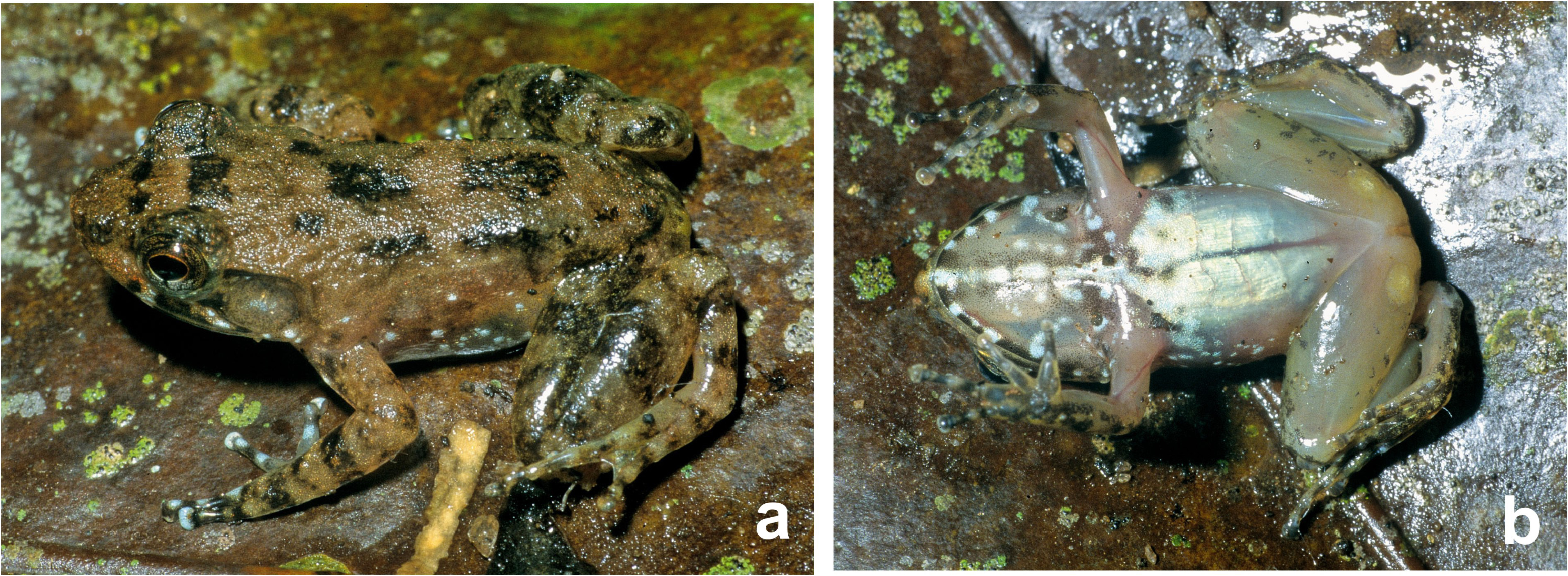
Variation.—Variation in measurements is given in Table 6 View TABLE 6 . See Fig. 31 View FIGURE 31 for colouration in life. There is moderate sexual size dimorphism (confirmed male SVL 17.3–22.3 mm [n = 5] vs confirmed female SVL 21.9–28.2 mm [n = 6]). Males have a distinctly larger tympanum than females (HTD/ED ratio is 59–73% in females, 94– 114% in males). Compared to the holotype, in other male specimens the femoral glands were smaller ( Fig. 31 View FIGURE 31 ), and smaller than in the sister species M. schulzi . In life the glands have a slightly yellowish tone ( Fig. 31 View FIGURE 31 ).
Natural history.—All specimens were observed in small streams and brooks in primary rainforest. Calling males were observed from one headwater pool, calling from positions directly next to the water during the day.
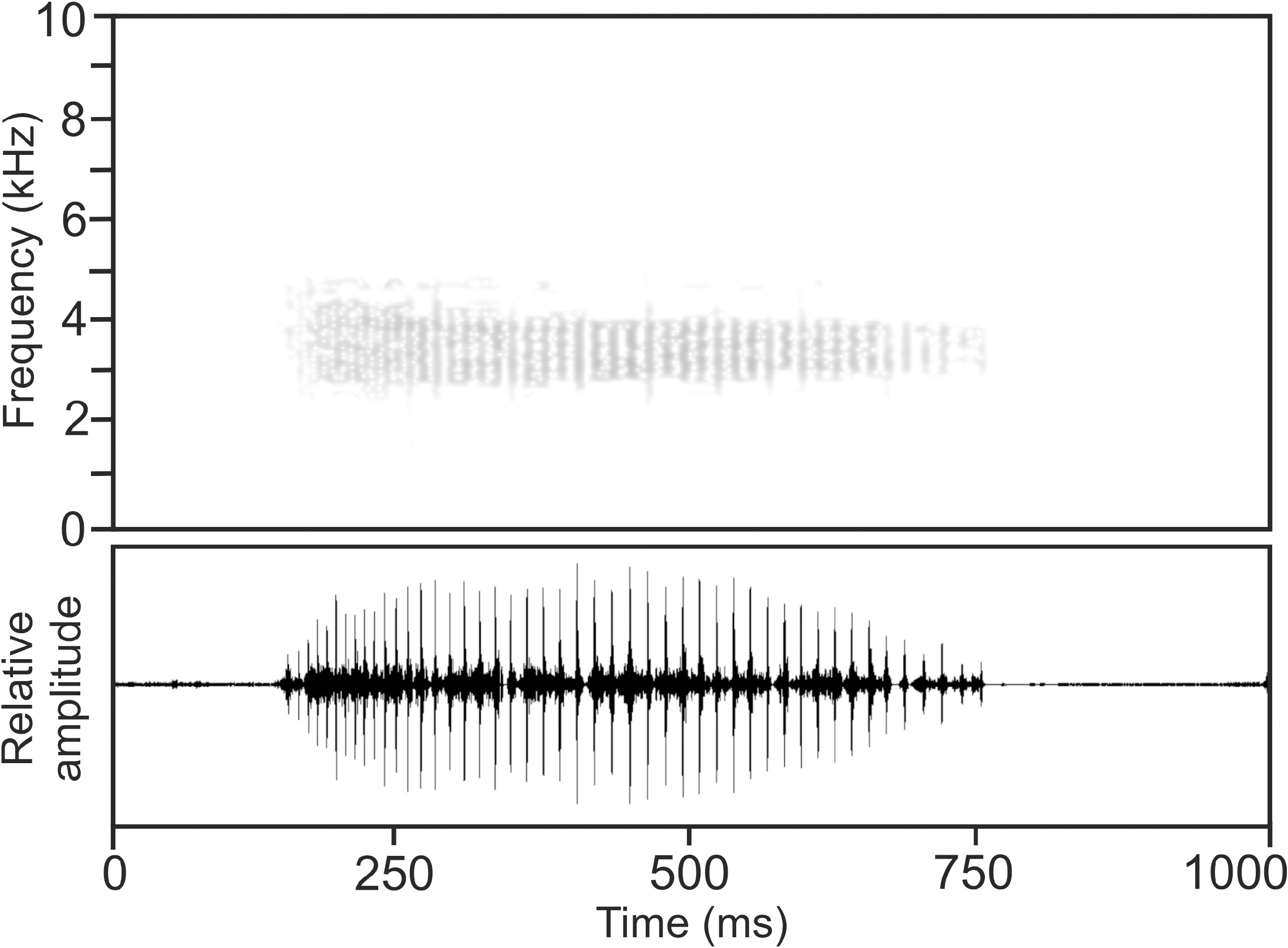
Calls.— The advertisement call recorded on 4 February 2001 at Antsahamanara Campsite, Manarikoba forest, Tsaratanàna Strict Nature Reserve, 20°C air temperature ( Vences et al. 2006: CD2, track 73), consists of a pulsed note ( Fig. 32 View FIGURE 32 ), emitted in groups containing two calls. Notes exhibit slight amplitude modulation, with maximum call energy occurring at approximately the middle of the note’s duration. Pulse repetition rate within notes is higher at the beginning of the note and slightly decreases after approximately the first quarter of the note’s duration. Numerical parameters of six analysed calls were as follows: call duration (= note duration) 516–721 ms (615.3 ± 87.8 ms); 40–54 pulses per note (47.2 ± 5.1); pulse duration 2–5 ms (2.9 ± 1.0 ms); pulse repetition rate within notes 69.8– 115.4 pulses/s (87.2 ± 17.5); dominant frequency 3193– 3716 Hz (3416 ± 184 Hz); prevalent bandwidth 2700–4300 Hz; call repetition rate (= note repetition rate) within call groups ca 24–36 calls/min.
Tadpoles.— The tadpole of this species has not been described.
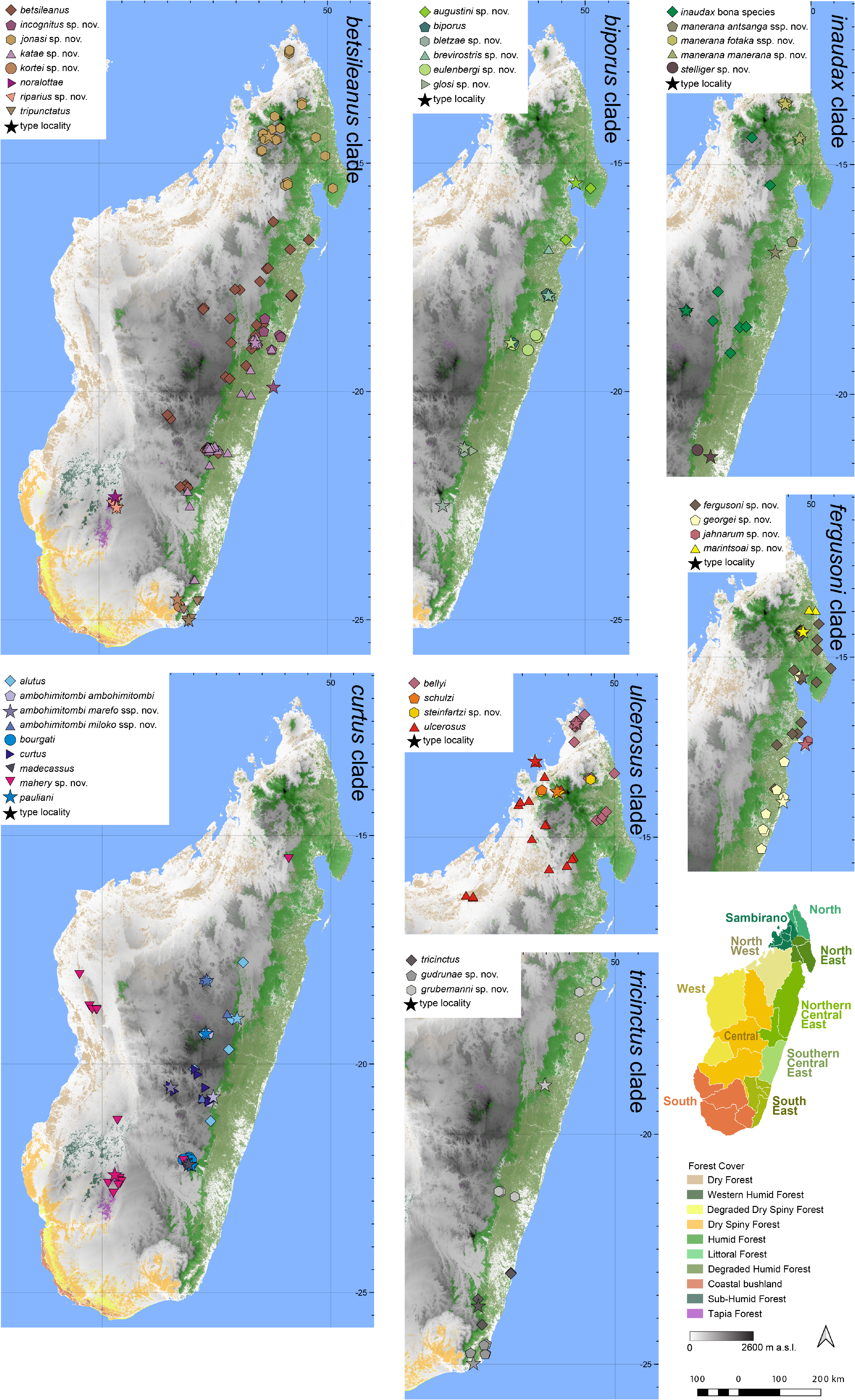
Distribution.— Endemic to the Sambirano Region in northern Madagascar ( Fig. 7 View FIGURE 7 ). This species is known from Tsaratanàna (Manarikoba, type locality), Manongarivo, and Ambodimandresy. Elevation range: 751–1000 m a.s.l.
Etymology.—We dedicate this species with an apparent ecological (elevational) component in species formation to our colleague Sebastian Steinfartz, in recognition of his contributions to the field of ecologydriven population differentiation and speciation
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |