Mantidactylus pauliani Guibé, 1974
|
publication ID |
https://doi.org/ 10.11646/megataxa.7.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:2FD8C310-6486-4592-92F6-5EB894EBD6AC |
|
DOI |
https://doi.org/10.5281/zenodo.7504352 |
|
persistent identifier |
https://treatment.plazi.org/id/5F25F715-FF91-FF9E-4CB1-4D4F4C927E6E |
|
treatment provided by |
Plazi |
|
scientific name |
Mantidactylus pauliani Guibé, 1974 |
| status |
|
Mantidactylus pauliani Guibé, 1974 View in CoL
Type material.— Mantidactylus pauliani Guibé, 1974 is based on the holotype (by original designation) MNHN 1972.1508 from ‘ Nosiarivo (massif d’ l’Ankaratra)’. There are eight paratypes ( Vences & Glaw 1999): MNHN 1972.1509–1516 .
Identity.— Mantidactylus pauliani is a morphologically distinct and apparently microendemic species restricted to high elevations on the Ankaratra Massif. Its identity has been assessed by Vences and Glaw (1999) and is unambiguous due to its microendemic distribution and typical short-snouted appearance. Therefore, no genetic data from the name-bearing type were collected.
Diagnosis.—A member of the M. curtus clade and sister to M. mahery sp. nov. (described below), from which it strongly differs morphologically. See Table 4 View TABLE 4 for a list of diagnostic morphological characters. The combination of a body size of 25–34 mm ( Table 5 View TABLE 5 ), small tympanum size in males (8–9% of SVL), smooth dorsal skin without dorsolateral ridges, absence of vomerine teeth, and strongly expressed foot webbing with fully webbed fifth toe distinguishes M. pauliani from species of the other clades in Brygoomantis . Within the M. curtus clade, this high-elevation endemic differs from all species exept M. curtus , M. madecassus , and M. ambohimitombi marefo , by a conspicuously short snout in most specimens, from M. madecassus by the single (vs bilobed) subarticular tubercles, and from M. a. marefo by absence of a bluish ring around the eye. Mantidactylus pauliani is endemic to high elevations at the Ankaratra Massif, where it is sympatric with M. a. ambohimitombi , which differs by larger body size, more pointed snout,and more contrasted dorsal pattern. For detailed distinction from new species described herein, see the respective species accounts. A full list of molecular diagnostic sites in the 16S gene of M. madecassus in pairwise comparisons to all other Brygoomantis species is provided as Supplementary appendix.
Variation.—Variation in measurements is given in Table 5 View TABLE 5 . See Fig. 20 View FIGURE 20 for colouration in life and its variation. There is weak sexual size dimorphism (confirmed male SVL 29.5–31.0 mm [n = 2] vs confirmed female SVL 31.1–33.7 mm [n = 4]), and males have a slightly larger tympanum diameter than females ( Vences & Glaw 1999). This is consistent with weak sexual size dimorphism reported by Andreone et al. (2014). Based on formalin-fixed and well-preserved voucher specimens of the MNHN collection, Vences and Glaw (1999) illustrate femoral glands in internal view, and document that females have weakly developed glands that are reminiscent in structure of those of males but with overall smaller gland granules. This same phenomenon of relatively well-developed gland rudiments in females, may also apply to several other species in the M. curtus clade and could make it difficult to sex preserved individuals. Future studies should assess whether femoral gland prominence in these frogs might also be influenced by seasonal effects.
Natural history. —Specimens were found sitting in the water or on exposed rocks in montane streams both inside and outside of forest (see Vences et al. 2002 for more information). Mantidactylus pauliani is rarely encountered and considered highly threatened ( Andreone et al. 2005). Age structure, population estimate, and status of infection with Batrachochytrium dendrobatidis were studied by Andreone et al. (2014). They found adult specimens ranging 3–8 years old, with no significant difference in age between males and females. Specimens reach sexual maturity in the second year in males and third year in females. Chytrid was not identified in these frogs.
Calls.—The call of this species has not been recorded.
Tadpoles.— The tadpole of this species has not yet been described in detail.
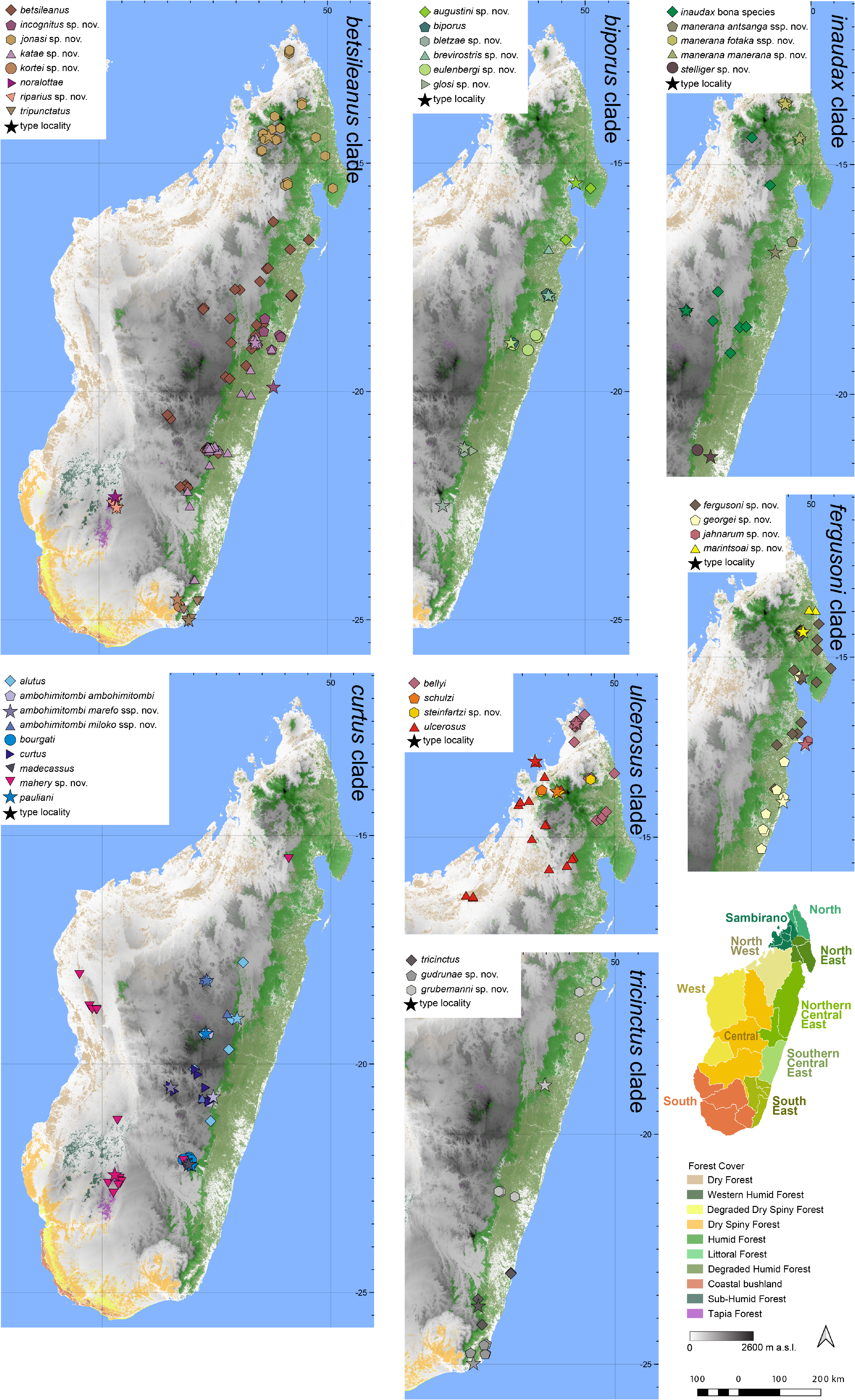
Distribution.— Apparently microendemic to high elevations on the Ankaratra massif ( Fig. 7 View FIGURE 7 ). Elevation range: all verified sites are from> 2000 m a.s.l. (up to at least 2200 m a.s.l.) (see Vences et al. 2002 for more information).
Etymology.— Eponym for R. Paulian, who initiated and directed the CNRS programme ‘Study of montane ecosystems in the Malagasy region’ (RCP 225) (loosely translated from Guibé 1973b).
| MNHN |
France, Paris, Museum National d'Histoire Naturelle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |