Macrostomum spiriger Wang & Xin, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4603.1.5 |
|
publication LSID |
lsid:zoobank.org:pub:0E1A44A9-C958-4869-9023-EB3086E679E7 |
|
persistent identifier |
https://treatment.plazi.org/id/03B387CD-AC6A-FF9E-FF4C-FC0DFA96FF17 |
|
treatment provided by |
Plazi |
|
scientific name |
Macrostomum spiriger Wang & Xin |
| status |
sp. nov. |
Macrostomum spiriger Wang & Xin View in CoL , n. sp.
( Figs. 5–7 View FIGURE 5 View FIGURE 6 View FIGURE 7 )
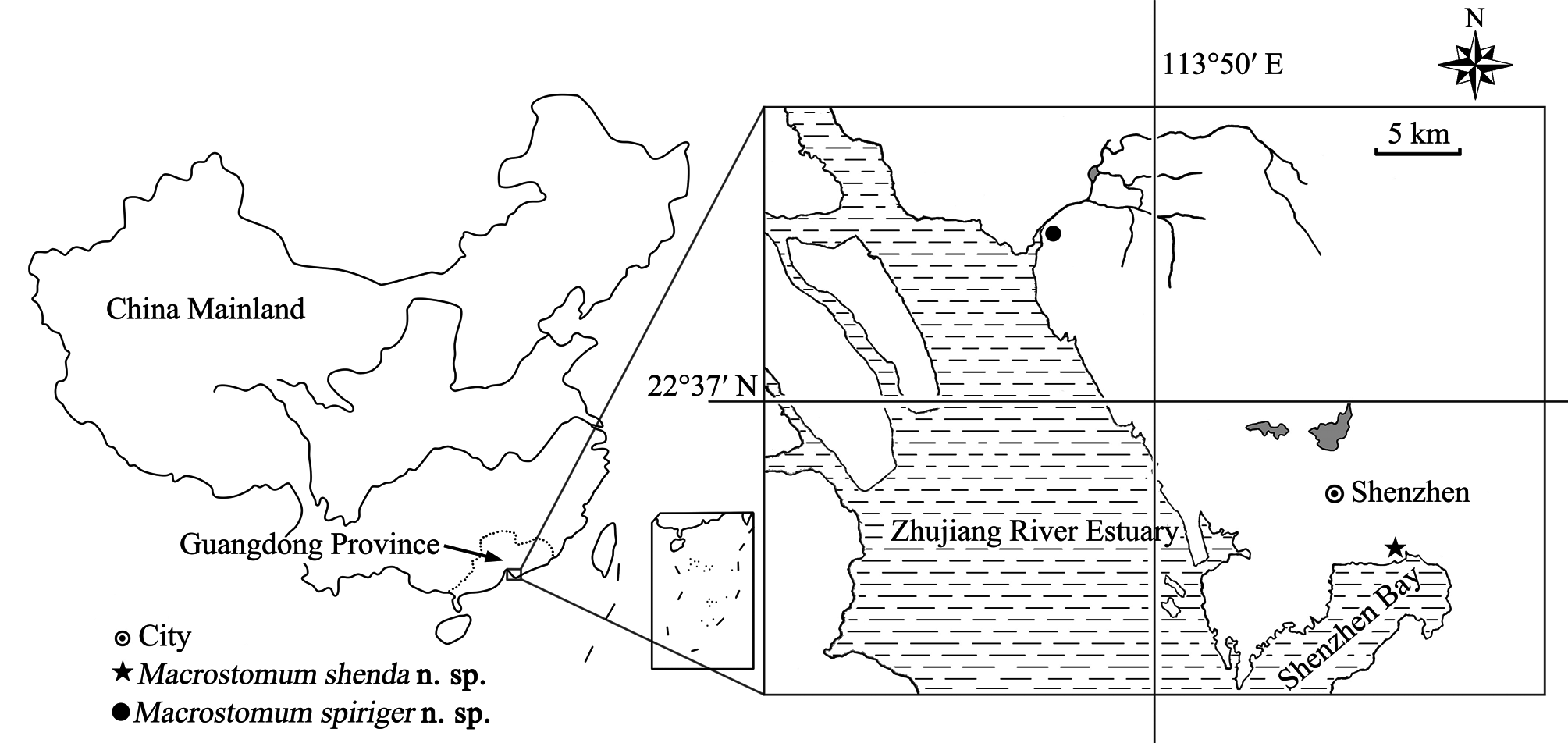
Material examined. Holotype ( PLA–Ma0100 ): one mounted specimen . Paratypes ( PLA–Ma0101–104 ): four serially-sectioned specimens. The type specimens were collected from Waterlands Resort , Shenzhen City, Guangdong Province, China (22°42′39″ N, 113°46′48″ E) (see Fig. 1 View FIGURE 1 for sampling location) in March, 2017. All specimens are deposited in IZCAS GoogleMaps .
Etymology. The name of this new species is derived from the morphology of its spiral-shaped penis stylet.
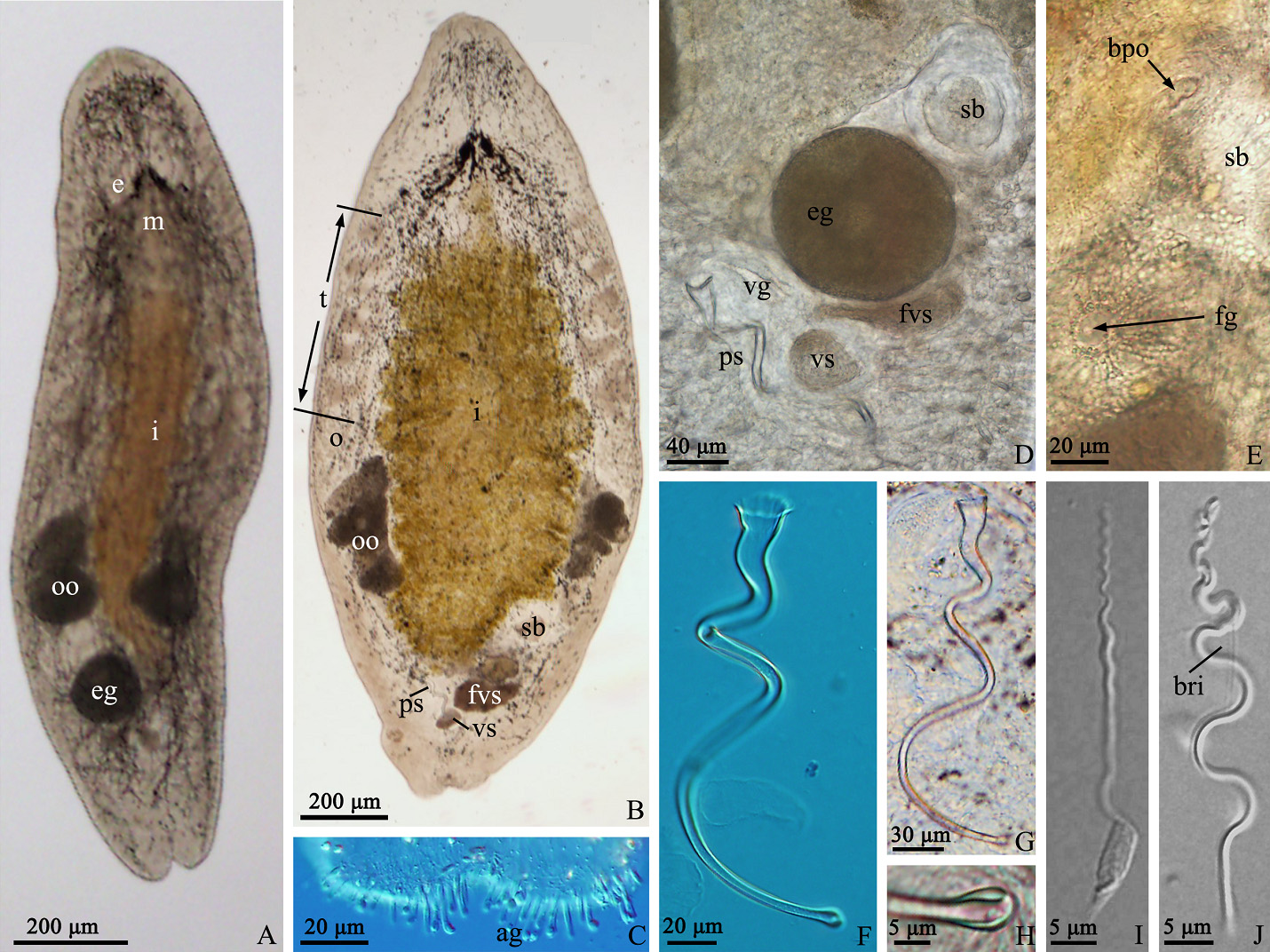
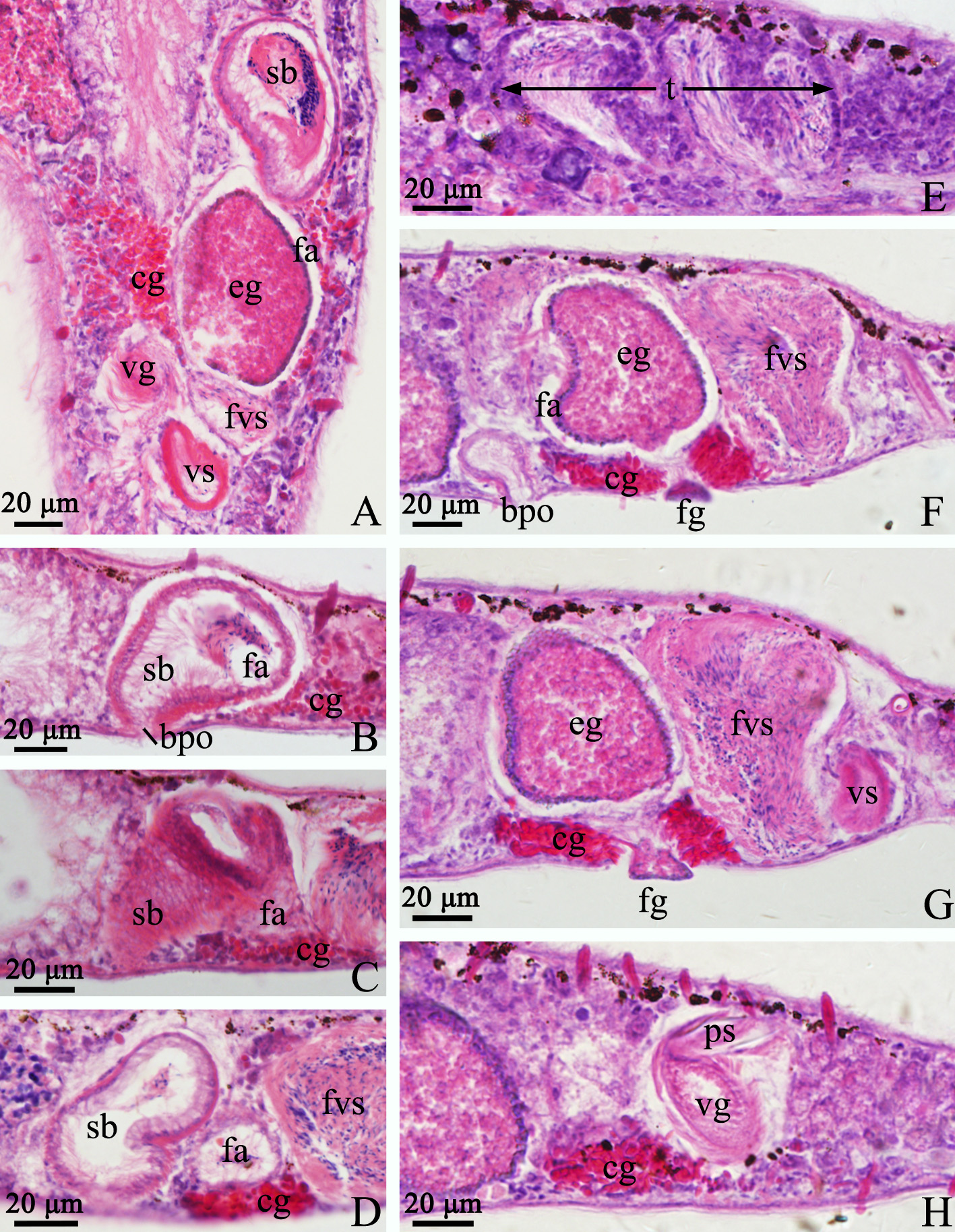
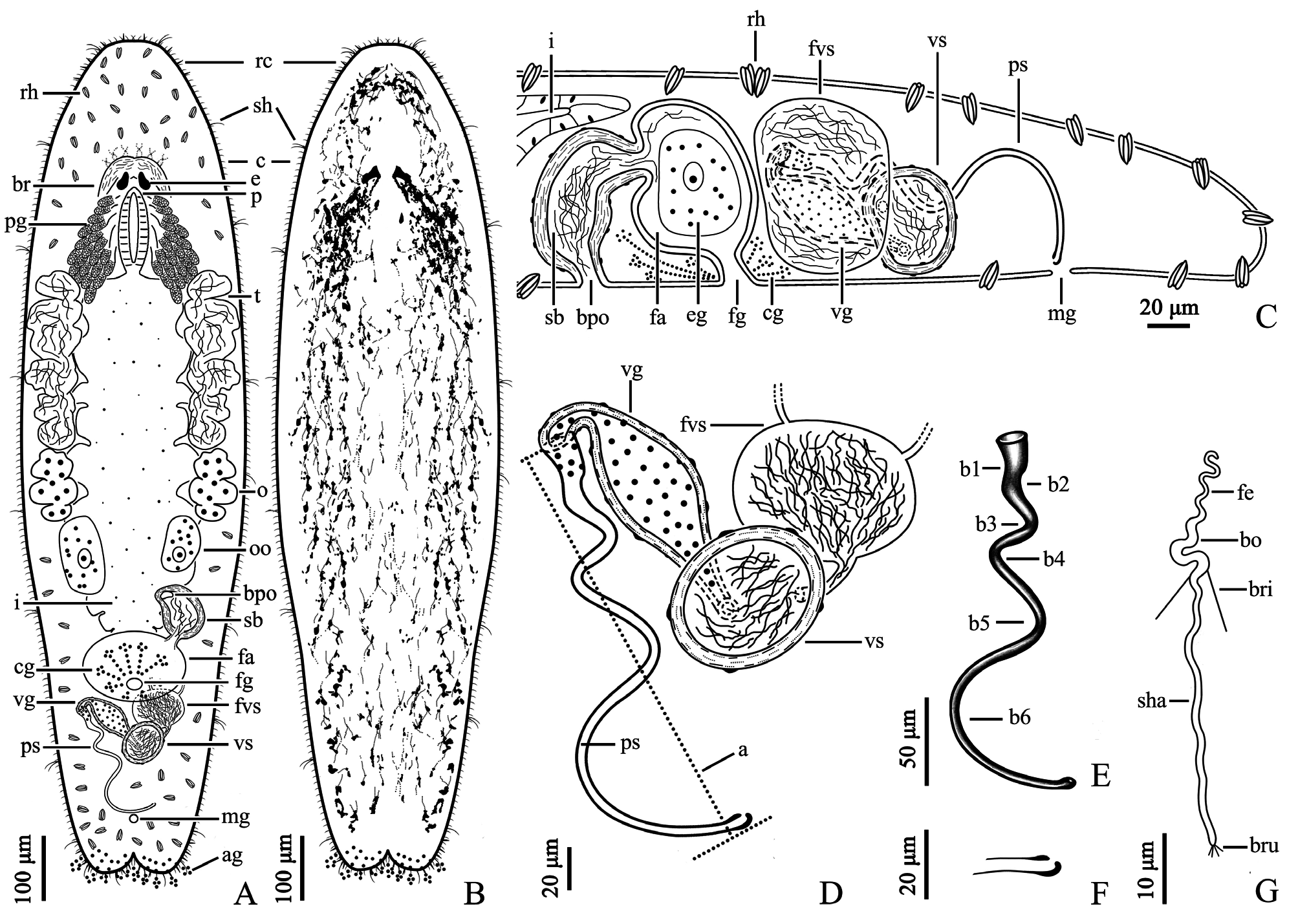
Description. The body is dorsoventrally flattened and the dorsal epidermis is covered by an irregular mesh of black pigments in different densities (particularly dense around the eyes). The tail is bifurcated ( Figs. 5 View FIGURE 5 A–B, 7A– B). The living mature individual is 1324 ± 104 µm (n=5) in length and 368 ± 37 µm (n=5) in width. The epidermal thickness varies up to 6 ± 1.2 µm with cilia up to 7 ± 1.0 µm (n=5). The rigid cilia, 8 ± 2.1 µm (n=5) in length, are present at both ends of the body. The sensory hairs are 24 ± 5.2 µm (n=5) long. The rhabdite rods, 20 ± 2.0 µm (n=5) long, are scattered in groups (mostly 1–3 rhabdites in each group) all over the body surface, and are particularly abundant on the dorsal surface. The distance between the two eyes is 43 ± 7.4 µm (n=5) ( Fig. 5 View FIGURE 5 A–B). The mouth is 106 ± 19.8 µm (n=5) in length. The pharynx is located posterior to the eyes and is surrounded by gland cells ( Fig. 7A View FIGURE 7 ). The tail region is abundant in adhesive glands ( Fig. 5C View FIGURE 5 ).
The paired testes, 266 ± 35.9 µm in length (n=5), are highly lobulate and generally contain 5 to 6 lobes on each side ( Figs. 5B View FIGURE 5 , 6E View FIGURE 6 , 7A View FIGURE 7 ). The false vesicula seminalis lies posterior to the female antrum and connects to the vesicula seminalis. The vesicula seminalis connects to the spindle-shaped vesicula granulorum via a short duct, while a small part of the vesicula granulorum is enclosed by the proximal end of the stylet ( Figs. 5D View FIGURE 5 , 6A View FIGURE 6 & F–H, 7C–D). Both the vesicula seminalis and vesicula granulorum have a thick muscular wall. The penis stylet, 270 ± 22.5 µm (n=5) in length, is spirally twisted. The straight line (marked as ‘a’) between the proximal and distal ends of the stylet is 184 ± 13.5 µm (n=5) ( Figs. 5 View FIGURE 5 F–G, 7D–E). The diameter of the proximal end is 16 ± 2.0 µm (n=5). The distal opening is 3 ± 0.7 µm (n=5) in diameter and is bulbous with an asymmetrical thickening ( Figs. 5H View FIGURE 5 , 7 View FIGURE 7 E–F).
Mature sperm are 72 ± 3.6 µm long (n=5) when swimming ( Figs. 5 View FIGURE 5 I–J, 7G). A pair of bristles, 10 ± 0.6 µm (n=5) long, lies on the sperm body. The extensions of the brush are 2 ± 0.3 µm (n=5) long. The lengths of the feeler, body and shaft of the sperm are 14 ± 2.1 (n=5), 13 ± 1.3 (n=5) and 46 ± 2.3 µm (n=5), respectively.
The female reproductive system consists of a pair of ovaries, a seminal bursa, a bursal pore, a female antrum and a female gonopore. A pair of indented ovaries, 110 ± 23.1 µm (n=5) long, is located posterior to the testes on both sides. The ovoid seminal bursa, 87 ± 10.5 µm (n=5) long, is located posterior to the left side of the intestine ( Figs. 5B View FIGURE 5 , 7A View FIGURE 7 ). The seminal bursa has a thickened muscular wall, and contains plenty of received sperm. It opens anteroventrally to the outside and connects to female antrum via a short, narrow duct ( Figs. 5 View FIGURE 5 D–E, 6B–D). The female antrum is surrounded by a numerous of cement glands.
Remarks. According to Ferguson (1954), in Macrostomidae , only two genera possess two openings to the exterior in their female reproductive system, one is the genus previously named Axia and the other is the genus Promacrostomum . The major difference between these two genera is that Axia lacks the structure “ductus-genitointestinalis ( An-der-Lan 1939)”, which is present in the genus Promacrostomum . For this reason, species Macrostomum gieysztori Ferguson, 1939 had been moved to Axia by Ferguson (1954) and was the only species in this genus. However, since the generic name Axia had not been accepted in the literature, Sluys (1986) suggested to assign species gieysztori to the genus Promacrostomum . Moreover, Schärer et al. (2011) noted that the generic name Axia had been occupied by a genus of Lepidoptera since 1821. Based on molecular phylogenetic analysis, Schärer et al. (2011) suggested to reinstate M. gieysztori to the genus Macrostomum .
The female reproductive system of M. spiriger n. sp. is similar to M. gieysztori in that they both have two exterior openings (female gonopore and bursal pore) and lack “ductus-genito-intestinalis”. M. spiriger n. sp. also clusters within the genus Macrostomum in 18S and 28S rDNA molecular phylogenetic analyses ( Figs. 8–11 View FIGURE 8 View FIGURE 9 View FIGURE 10 View FIGURE 11 ), therefore, we classified M. spiriger n. sp. into Macrostomum .
In summary, the stylet of M. spiriger n. sp. is spirally twisted with six bends (b1–b6, Fig. 7E View FIGURE 7 ), which is a unique morphological characteristic within the genus Macrostomum , and this species has a seminal bursa located anterior to the female gonopore. As such, it is evident that M. spiriger n. sp. is a new species within Macrostomum .
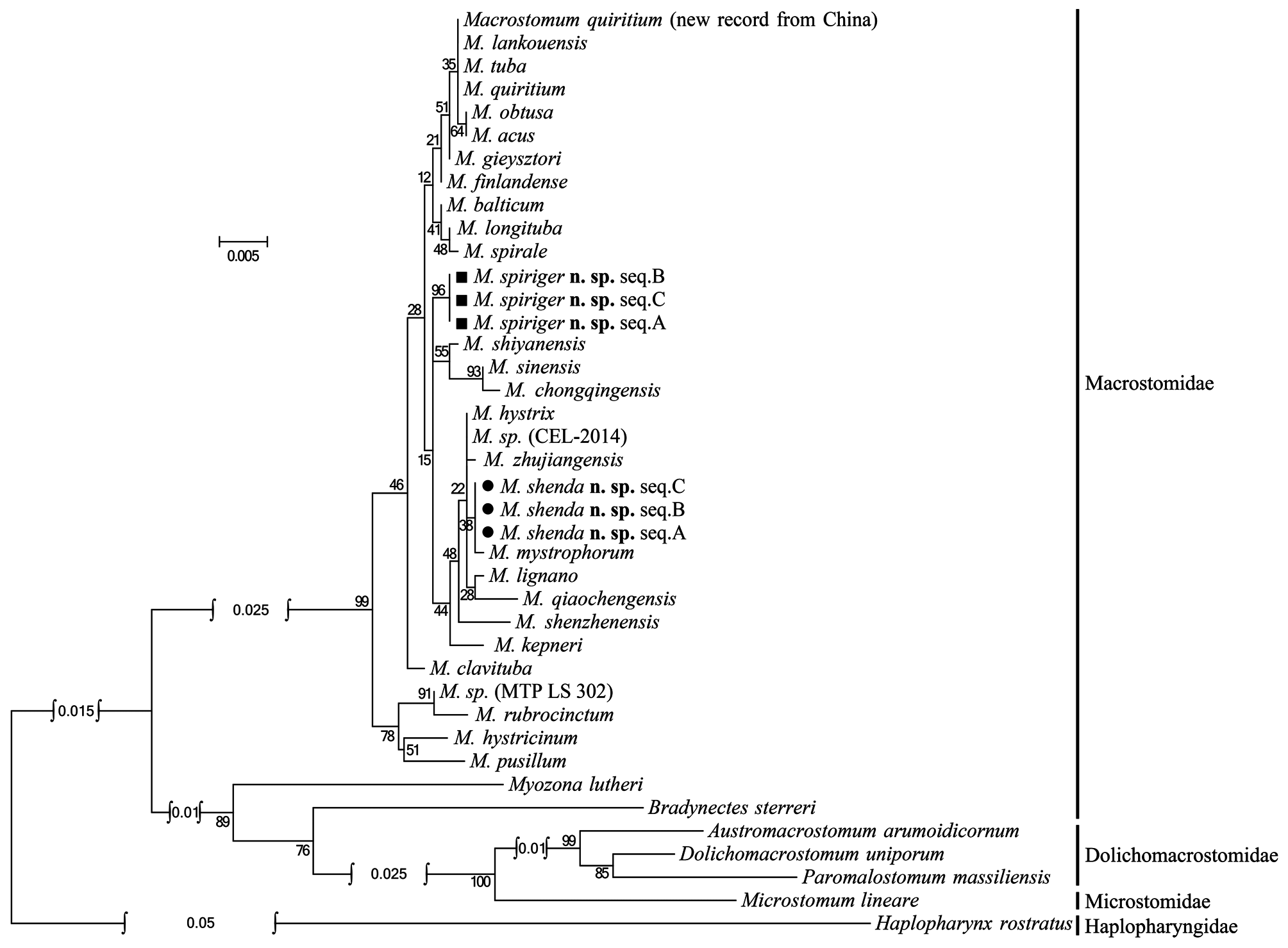
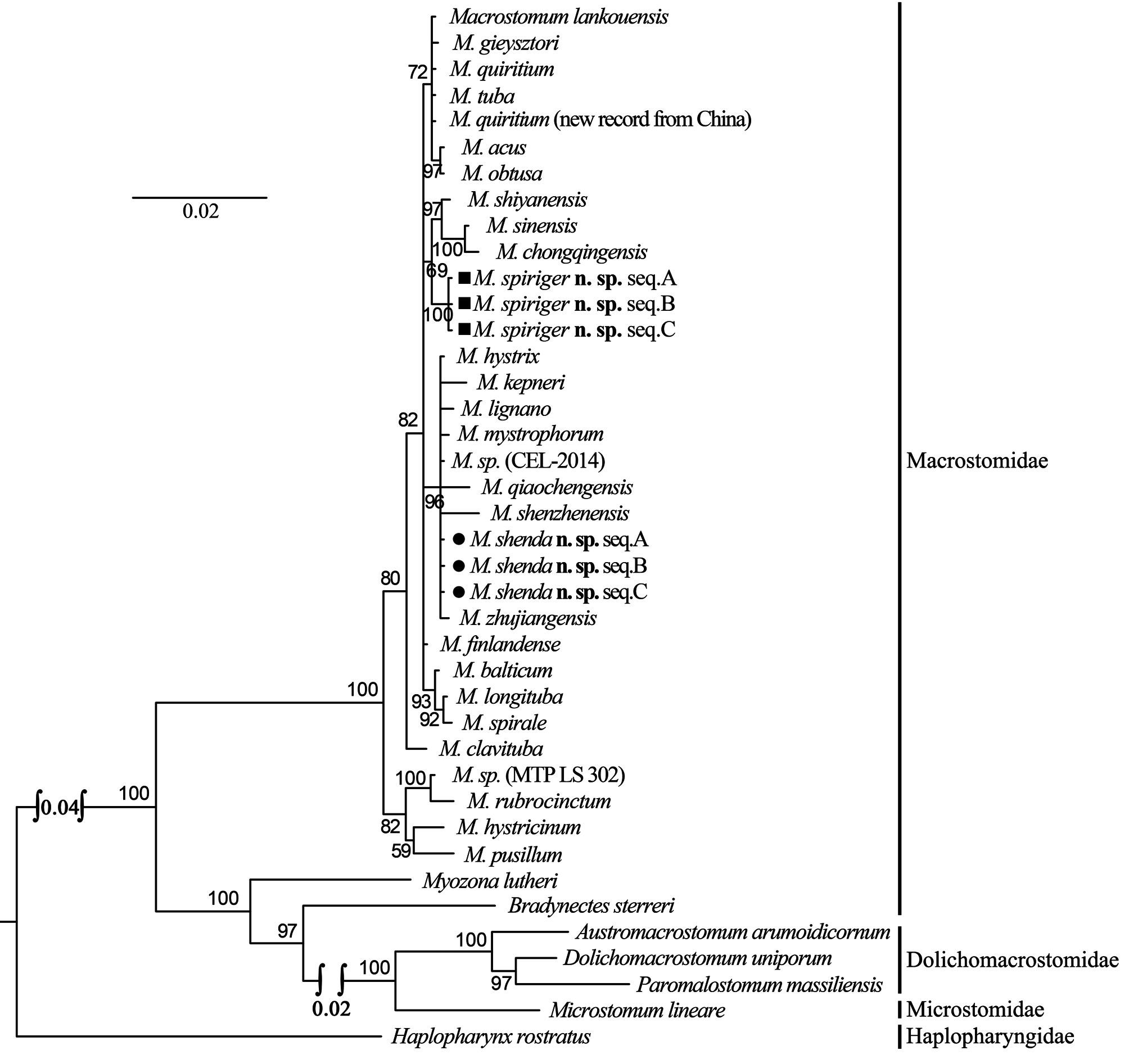
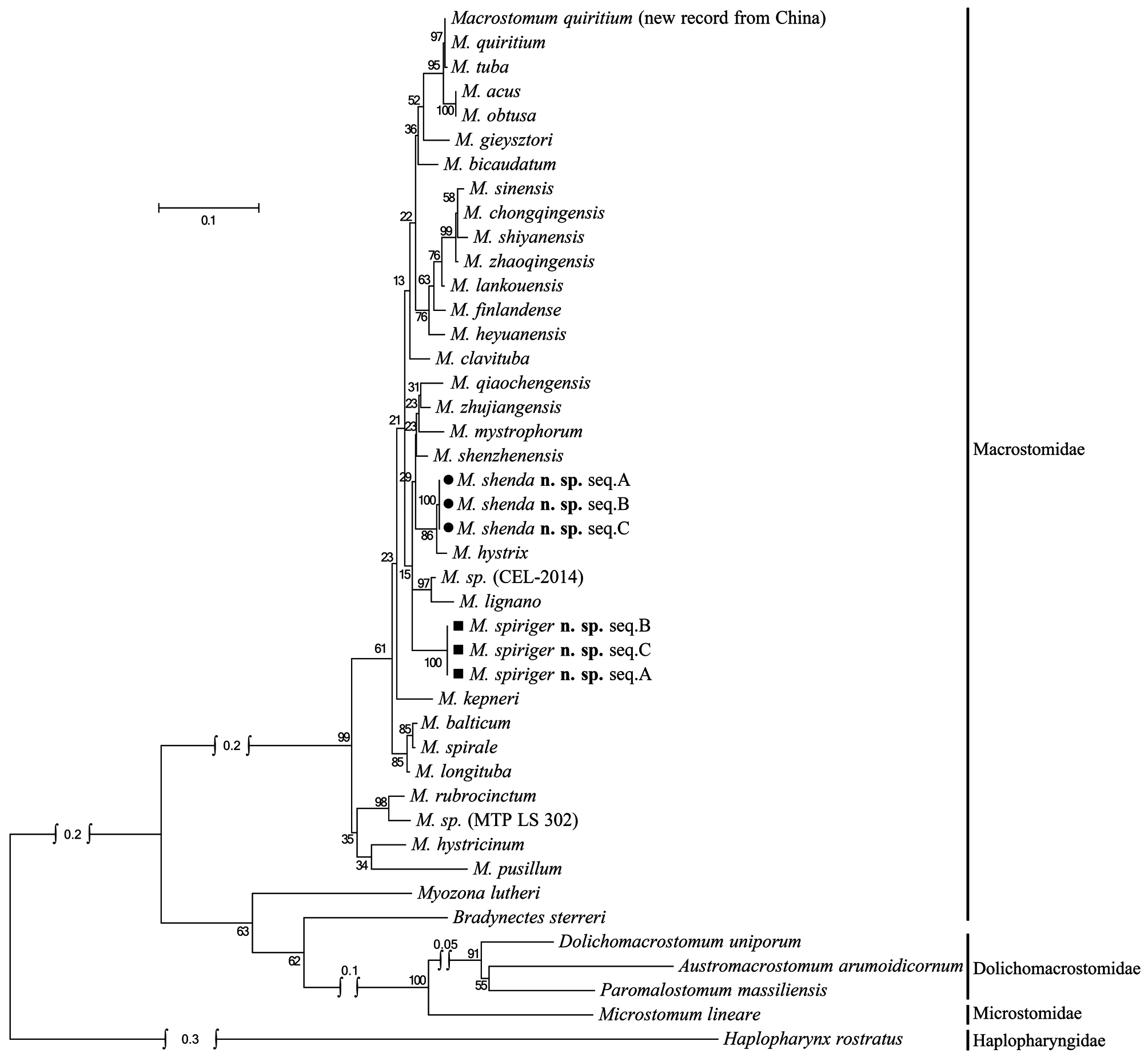
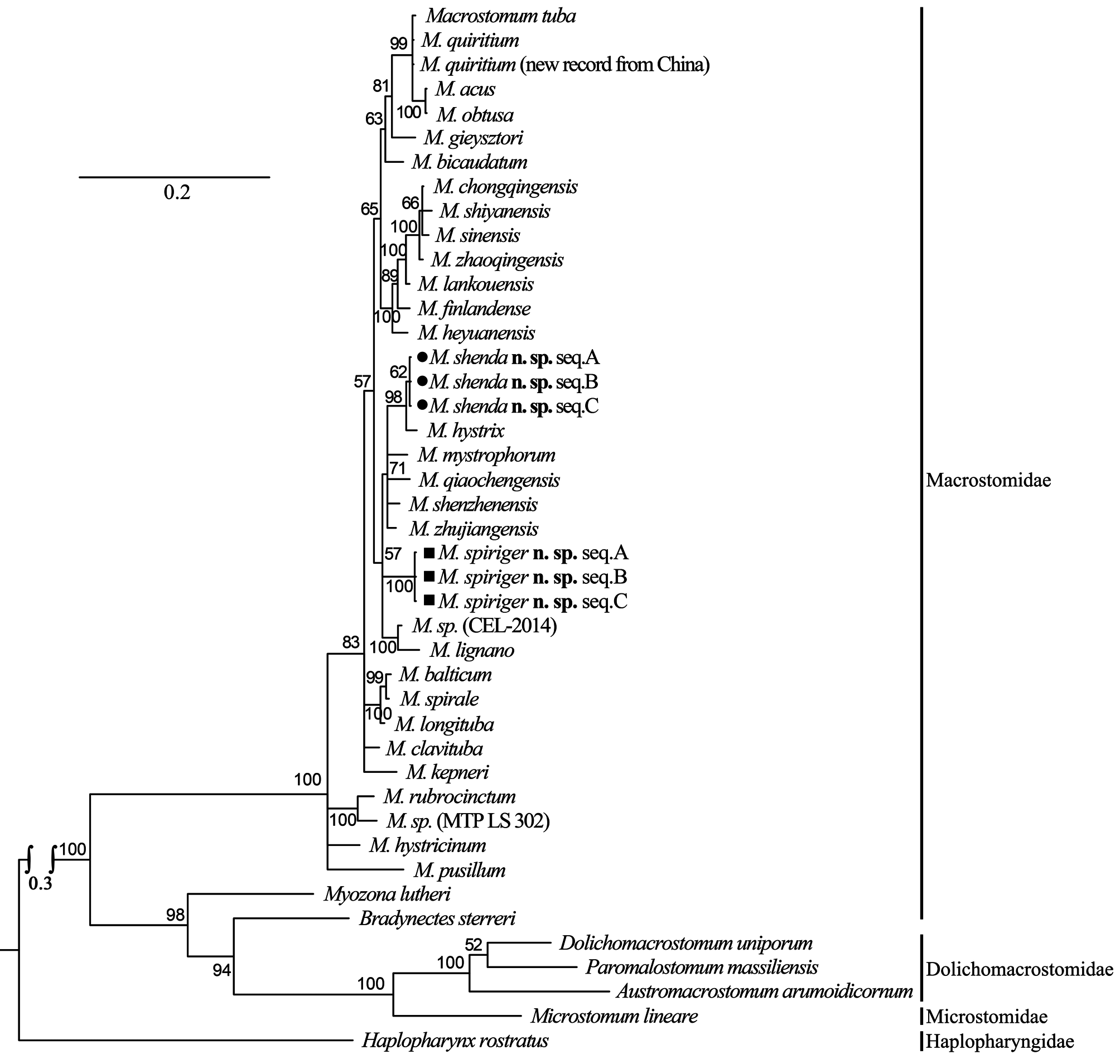
Molecular phylogenetic analysis. The phylogenetic trees generated from single gene (18S and 28S rDNA, respectively) by Maximum Likelihood (ML) and Bayesian-inference (BI) methods are shown in Figs. 8–11 View FIGURE 8 View FIGURE 9 View FIGURE 10 View FIGURE 11 . The 28S rDNA results indicated that the three specimens of the new species cluster together respectively, forming a well-supported clade with other species within the genus Macrostomum . Although these two species are clearly separated by their 28S rDNA sequences and morphological characters, M. shenda n. sp. cannot be well-separated based on 18S rDNA analyses. Similar situation can also be found in other species of the genus, including M. tuba and M. quiritium ( Figs. 8 View FIGURE 8 , 9 View FIGURE 9 ). As such, both the 28S rDNA phylogenetic and morphological evidence supports the establishments of M. shenda n. sp. and M. spiriger n. sp. as new species within the genus Macrostomum .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SuperOrder |
Macrostomorpha |
|
Order |
|
|
Family |
|
|
Genus |