Janstockia phallusiella, Boxshall & Marchenkov, 2005
|
publication ID |
https://doi.org/ 10.5281/zenodo.5398409 |
|
persistent identifier |
https://treatment.plazi.org/id/0395CB44-FFEB-FFFC-5F94-EFB2FBBFFDC6 |
|
treatment provided by |
Marcus |
|
scientific name |
Janstockia phallusiella |
| status |
sp. nov. |
Janstockia phallusiella n. sp.
( Figs 1-6 View FIG View FIG View FIG View FIG View FIG View FIG ; 7A, B View FIG ; 9 View FIG )
TYPE MATERIAL. — Holotype and 8 paratype (1 incomplete). Three females dissected on slides, two prepared for SEM on stubs. Registration numbers: holotype MNHN-Cp2177, two paratypes (one in alcohol and one dissected on 3 slides) MNHN- Cp2178, MNHN-Cp2179; five paratypes (one in alcohol, two on SEM stubs and two dissected on 2 and 3 slides respectively) BMNH 2004 .247-251; one paratype in alcohol, Zoological Museum, St Petersburg, Reg. No. 18096.
TYPE LOCALITY. — Suez Canal.
TYPE HOST. — Phallusia nigra Savigny, 1816 (site in host unknown).
ETYMOLOGY. — The specific name is derived from the generic name of the host ascidian.
DESCRIPTION
Based on adult female, male unknown.
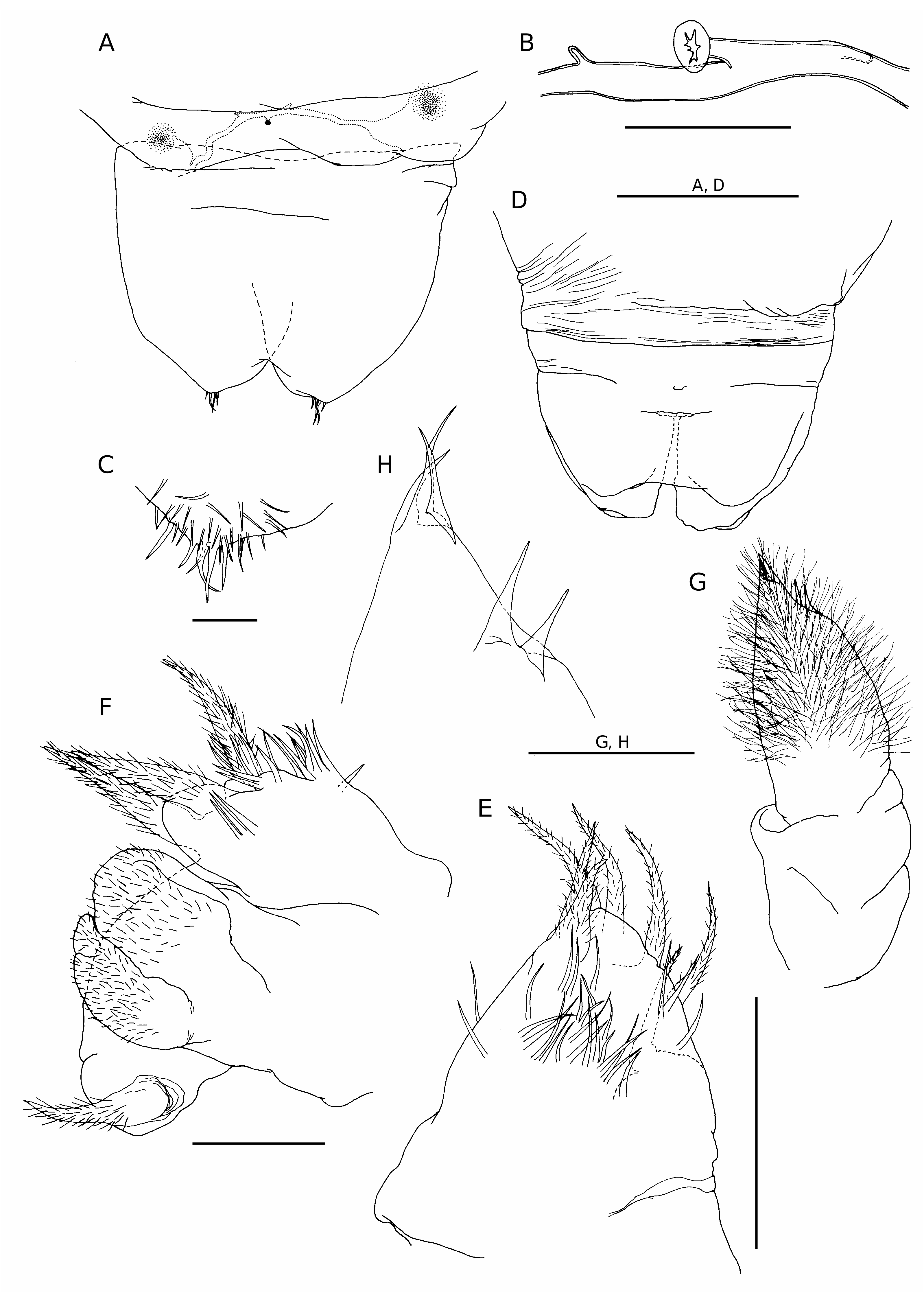
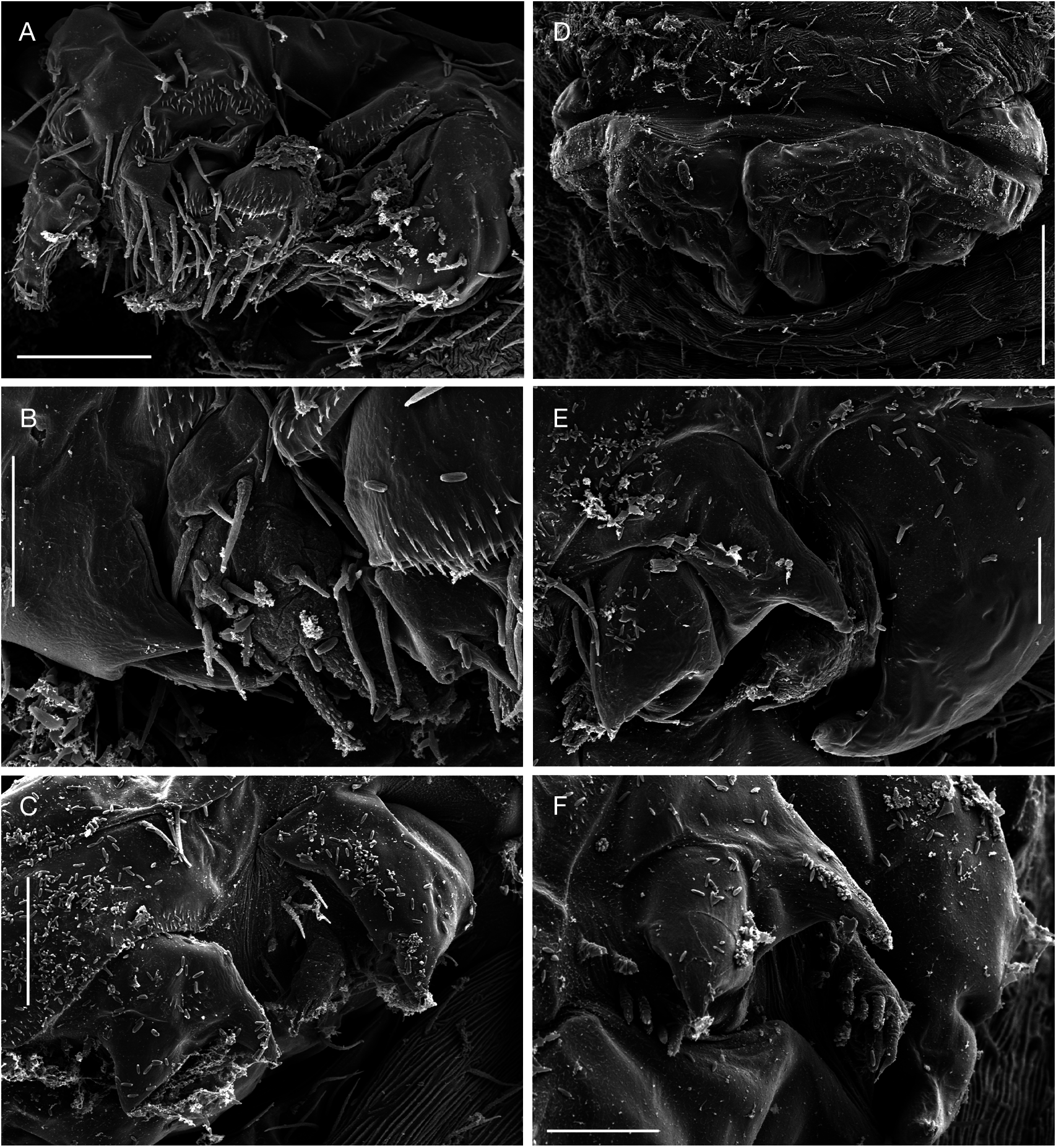
Adult female vermiform ( Fig. 1 View FIG ), comprising distinct head and elongate, postcephalic trunk terminating in small abdomen ( Fig. 5A, D View FIG ). Mean body length 6.2 mm, with range from 5.1 to 8.4 mm. Head somewhat dorsoventrally flattened, with flared swollen posterolateral margins partly concealing lateral expansions of first pedigerous somite (arrowed in Figure 1C View FIG ). Swollen lateral margins extending medially to partly conceal mouthparts ( Figs 1B View FIG ; 3A View FIG ). Rostrum well developed, fused to dorsal cephalic shield; connected to anterior margin of labrum by mid-ventral ridge ( Fig. 3B View FIG ). Entire surface of dorsal cephalic shield and anterior part of rostrum densely ornamented with hairlike setules ( Fig. 3A, B View FIG ). Postcephalic trunk comprising first to fifth pedigerous somites but without clearly defined segmental boundaries. First pedigerous somite shortest, with paired lateral expansions ( Fig. 1C View FIG ); second to fourth pedigerous somites elongate, with linear lateral margins. Surface of trunk highly ornamented with complex system of epicuticular ridges, mostly orientated transversely around the body; numerous hair-like setules distributed among these ridges ( Fig. 3C View FIG ). Swimming legs 1 to 4 located at approximately 1%, 5%, 24% and 60% of length of trunk, respectively ( Fig. 1A View FIG ). Each leg pair carried on slightly raised, common, ovoid pedestal, which is not ornamented with surface ridges and appears to be retractable into body ( Figs 3A View FIG ; 6D View FIG ). Fifth leg located laterally just anterior to boundary zone where trunk tapers down to abdomen. Genital field with midventral copulatory pore ( Figs 3D View FIG ; 5A, B View FIG ) and paired internal ducts; genital field lacking ornamentation of surface setules ( Fig. 3D View FIG ). Abdomen indistinctly two-segmented, terminating in paired rounded lobes representing caudal rami incorporated into urosome, each armed with five or six caudal setae ( Fig. 5C View FIG ) (setae difficult to distinguish from numerous surface setules).
Antennule conical ( Fig. 2A, B View FIG ), tapering strongly from broad base; posterior margin showing traces of original segmentation pattern. Setal formula indeterminable: setae largely concealed within dense covering of setules over anterior surface; three aesthetascs present distally ( Fig. 2B View FIG ).
Antenna ( Fig. 2C View FIG ) comprising robust proximal segment (coxobasis), lacking setal armature, and compound distal segment terminating in strong claw ( Fig. 2D View FIG ) and cluster of five setal elements (three terminal setae and two subterminal setae). Patch of tiny denticles present medially, near base of claw.
Labrum ( Fig. 3B View FIG ) consisting of an elongate, midventral lobe; naked anteriorly but densely ornamented with hair-like setules posteriorly.
Mandible ( Figs 4A View FIG ; 5E View FIG ) lacking gnathobase, consisting of palp only, represented by tapering, conical lobe armed with four setae terminally and three setae along anterior margin; all setae are hirsute; surface ornamented with numerous, slender setules.
Maxillule ( Figs 4B, C View FIG ; 5F View FIG ) complex in structure; consisting of three lobes of uncertain homology, arising from common base and arranged anteriorly to posteriorly. Anterior lobe simple, armed with one subterminal and two terminal setae; surface of lobe ornamented with setules. Middle lobe subdivided into two rounded processes covered with long hair-like setules ( Fig. 3C View FIG ). Posterior lobe simple, armed with one terminal seta. All setae hirsute.
Maxilla very reduced, represented by small, unarmed, digitiform lobe located posteromedially to base of maxillules (arrowed in Figure 2E View FIG ).
Maxilliped ( Figs 4D View FIG ; 5G View FIG ) elongate, two- segmented; proximal segment cylindrical, without setation or ornamentation: distal segment densely covered with long hair-like setules and armed with three weak setae terminally and two subterminally ( Fig. 5H View FIG ).
First to fourth pairs of legs ( Figs 6 View FIG ; 7A, B View FIG ) with broad, ovoid protopodal part common to both members of leg pair; typically ornamented with patches of fine spinules and scattered, hair-like setules. Members of each leg pair connected by small but strongly sclerotized intercoxal sclerite. Legs 1 to 4 biramous, with modified, indistinctly segmented rami; outer protopodal (basal) seta present on all legs. Legs apparently retractable into body.
Leg 1 ( Figs 6A, B View FIG ; 7A View FIG ) with large spinulate inner basal seta and slender outer basal seta. Exopod indistinctly two-segmented: outer distal angle of first segment produced into pointed sclerotized process, armed with single outer spine ( Fig. 6B View FIG ); distal segment not sclerotized, cuticle wrinkled and ornamented with long setules; armed with five short setae distally; setae finely spinulate over surface and with rounded tips. Endopod indistinctly two-segmented; proximal segment ornamented with patches of fine spinules ( Fig. 6B View FIG ), distal segment broad, armed with similar short, blunt setae as on exopod, partly concealed by irregular ornamentation of large setules; array of integumental pores present near apex of endopod. Legs 2 to 4 ( Figs 6D, F View FIG ; 7B View FIG ) all similar in structure: biramous with exopod two-segmented, outer distal angle of first segment produced into pointed sclerotized process, lacking outer spine; distal segment cylindrical, cuticle not sclerotized, but wrinkled and ornamented with scattered long setules; armed with four, five or six short setae distally; setae finely spinulate over surface and with rounded tips. Endopod indistinctly segm e n t e d; p r o x i m a l p a r t p r o d u c e d i n t o t w o pointed sclerotized processes, probably derived from outer distal angles of first two endopodal segments, and a small raised lobe, representing third endopodal segment, carrying about five short setae with blunt tips. Third endopodal segment forming a weak incorporated lobe in leg 2 ( Fig. 6C View FIG ), leg 3 ( Fig. 6E View FIG ) and leg 4 ( Fig. 6F View FIG ).
Fifth leg represented by two setae inserted directly on body surface near posterior margin of trunk.
REMARKS
The new genus shares the vermiform body entirely covered with setular ornamentation with five other notodelphyid genera ( Ooishi 1998): Haplostatus Illg & Dudley, 1961 , Ophioseides , Pholeterides Illg, 1958 , Prophioseides Chatton & Brément, 1915 (excluding P. ampullacea Ooishi, 1972 , the generic affiliation of which is, according to Ooishi [1998], in need of reconsideration) and Pythodelphys Dudley & Solomon, 1966. In addition, the body of Anoplodelphys Lafargue & Laubier, 1978 is covered with setular ornamentation and tends towards a vermiform morphology. Prophioseides , Pholeterides and Pythodelphys are all characterized by extreme reduction or loss of swimming legs 1 to 4, while at the same time typically retaining a well developed, setose maxilla and a well developed mandible (with biramous palp and gnathobase). Anoplodelphys species exhibit reduced or transformed oral appendages and legs 1 to 4, when present, are typically biramous with the rami forming elongate lobes. Haplostatus has extreme reduction of the mouthparts and has no swimming legs. In contrast, the new genus and Ophioseides are characterized by reductions of the mouthparts (i.e. loss of the mandibular gnathobase and extreme reduction of the maxilla to a minute lobe) in combination with retaining well developed, although modified, swimming legs 1 to 4. These genera also share produced tergal margins of the first pedigerous somite, which form the lateral lobes lying just posterior to the cephalosome. The new genus can be distinguished from Ophioseides by the complex, trilobate form of the maxillule of the new genus (either absent or represented by a minute lobe in Ophioseides ) and by the two-segm e n t e d s t a t e o f t h e m a x i l l i p e d (a b s e n t i n Ophioseides ).
Ophioseides cardiocephalus Hesse, 1864 MATERIAL EXAMINED. — France. Dinard, in Dendrodoa grossularia (Van Beneden, 1846) , 2,
1 in alcohol (MNHN-Cp2180), 1 dissected. — Banyuls-sur-Mer, in Distomus variolosus Gaertner, 1774 , 3, 1 in alcohol, 1 dissected on 2 slides (BMNH 2004.245-246); 1 in alcohol (Zoological Museum, St Petersburg 275).
SUPPLEMENTARY DESCRIPTION OF ADULT FEMALE Body vermiform (as figured by Bocquet & Stock 1961: figs 1-4). Caudal rami incorporated into urosome and represented by vestigial lobes bearing five caudal setae terminally and ornamented with hair-like setules.
Antennule ( Fig. 8A, B View FIG ) an unsegmented tapering lobe, showing traces of segmentation in form of sclerotizations along posterior margin. Surface densely ornamented with hair-like setules concealing setal elements except around apex ( Fig. 8B View FIG ).
Antenna ( Fig. 8C, D View FIG ) comprising robust proximal segment (coxobasis) and compound distal segment representing endopod: distal segment with seta at mid-length, at plane of fusion between first and second endopodal segments; apical armature comprising claw, plus four stout setae.
Three pairs of oral appendages present ( Fig. 8E View FIG ). Anterior pair (mn, Fig. 8 View FIG ) represented by elongated slightly narrowing lobe armed with two long and one short terminal setae and one long subterminal seta. Posterior pair (mx, Fig. 8 View FIG ) represented by large unsegmented lobe armed with four long terminal setae and one long lateral seta. Vestige of third appendage (mxl, Fig. 8 View FIG ) located between bases of anterior and posterior pairs, forming tiny digitiform lobe with weak apical lobules. Maxillipeds absent.
First to fourth pairs of legs all similar ( Fig. 7C View FIG ), each comprising large ovoid protopod bearing rami distally. Exopod armed with two sclerotized hooks; endopod with single sclerotized hook: surfaces of all legs ornamented with fine setules and small spinules.
REMARKS
Establishing the homology of the three pairs of oral appendages is problematic. The anterior pair probably represents the mandibular palps (the gnathobase is lacking). The posterior pair probably represents the maxillae and their structure and setation is basically the same in all Ophioseides species. Illg & Dudley (1961, as Scolecodes ) labelled these two appendages “Md” and “Mx” respectively. The third paired structure has not been reported previously for any species of Ophioseides , although it may have been overlooked because of its small size and thin cuticle. We interpret it as probably representing the maxillule, on the basis of its position between the mandible and maxilla.
This species has a complex nomenclatural and taxonomic history. Ophioseides cardiocephalus was first described by Hesse (1864) under the vernacular binomen “ Ophioséide cardiocéphale” but was attributed to Bate, 1864 (Bate was the compiler of the Zoological Record for 1864) by Illg (1958) under the name Ophioseidus cardiocephalus . Even though it could be argued that Illg (1958) was acting as first reviser, Bocquet & Stock (1961) revisited this nomenclatural issue and concluded that the genus and species should be attributed to Gerstaecker (1870 -1871), as Ophioseides cardiacephalus . Their argument was that Hesse (1864) did not attribute a latinised name to his vernacular “ Ophioséide cardiocéphale” and that Gerstaecker (1870 -1871) was the first to give a diagnosis of the genus and mention the name Ophioseides cardiacephalus . The genus names Ophioseides and Ophioseidus were both proposed as latinised versions of Ophioséide , proposed by Hesse in 1864. Ophioseides was attributed to Hesse by Gerstaecker (1870 -1871). We therefore consider that there is no justification in attributing this genus to any author other than Hesse, 1864. Although Boxshall & Halsey (2004) used the original spelling Ophioseide , we consider that nomenclatural stability would be best served by adopting the spelling of Ophioseides as used by Gerstaecker (1870 -1871), since this has been widely used for over 100 years. We consider that there is no justification for changing the spelling of the specific epithet from cardiocephalus to cardiacephalus and we follow Illg (1958) in using the former. We therefore adopt the name Ophioseides c a r d i o c e p h a l u s H e s s e, 1 8 6 4 f o r t h i s t a x o n. Boxshall & Halsey (2004) mistakenly stated that Bocquet & Stock (1961) concluded that the genus should be attributed to Hesse, 1864 whereas they concluded it should be Gerstaecker (1870 -1871).
A second species Ophioseides joubini was established by Chatton (1909) based on material from the ascidian host “ Microcosmus sabatieri Roule, 1885 ” (possibly = Microcosmus sulcatus Coquebert, 1797 ) collected at Banyuls-sur-Mer. Subsequently Schellenberg (1922) described material from New Zealand (from the ascidians Pyura trita Sluiter, 1900 and Cnemidocarpa cerea Sluiter, 1900 ), which he attributed to Ophioseides joubini . In his monograph on the family, Illg (1958) recognised this taxon in a new combination, as Scolecimorpha joubini ( Chatton, 1909) . It is unclear why Illg choose to adopt the genus Scolecimorpha Sars, 1926 over Ophioseides . Later, Illg & Dudley (1961) published a detailed redescription of Scolecimorpha joubini using Chatton’s (1909) type material and other material from the type locality. They drew attention to the close relationship between the type species of the two genera, stating: “The type of the genus is Scolecimorpha insignis Sars, 1926 , which appears to us quite possibly conspecific with S. joubini , the older species” ( Illg & Dudley 1961: 87). They continued: “This question will probably have to be settled by study of topotypic material of Sars’ species from Norway ”.
In the same year a detailed redescription of Ophioseides joubini from the host Distomus vari- o l o s u s G a e r t n e r, 1 7 7 4 c o l l e c t e d i n B a i e d e Morlaix and Baie de St-Malo was published by Bocquet & Stock (1961). They reviewed the genus Ophioseides and demonstrated that Ophioseides joubini should not be placed in Scolecimorpha . They reverted to the original name Ophioseides joubini for this species. Comparison between Illg & Dudley’s (1961) redescription of Scolecimorpha joubini and Bocquet & Stock’s (1961) redescription of Ophioseides cardiocephalus leaves no doubt that they are conspecific. Subsequently, Stock (1967) included O. joubini as a junior synonym of O. c a r d i o c e p h a l u s. F i n a l l y, J o n e s (1 9 7 9) redescribed the material from New Zealand identified as O. joubini (Chatton) by Schellenberg (1922), on the basis of type and newly collected material from the host Pyura cancelata Brewin, 1946 from the type locality. He established a new species for this material, Ophioseides schellenbergi Jones, 1979 . There are currently three valid species of Ophioseides : O. cardiocephalus from European waters, O. elongatus Stock, 1967 from the Red Sea and O. schellenbergi from New Zealand. O. joubini Chatton, 1909 is a junior subjective synonym of O. cardiocephalus .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.