Ishimosorex ishimiensis, Zazhigin & Voyta, 2022
|
publication ID |
https://doi.org/ 10.26879/1209 |
|
publication LSID |
lsid:zoobank.org:pub:1726FDAE-2EE5-4145-A124-6D24287C0514 |
|
DOI |
https://doi.org/10.5281/zenodo.11105190 |
|
persistent identifier |
https://treatment.plazi.org/id/71755174-FFAE-0330-CE30-F918CBB9F892 |
|
treatment provided by |
Felipe |
|
scientific name |
Ishimosorex ishimiensis |
| status |
sp. nov. |
Ishimosorex ishimiensis sp. nov.
Figure 2 View FIGURE 2
zoobank.org/ DFBFD952-8B5C-4392-8522-867F4694C1E1
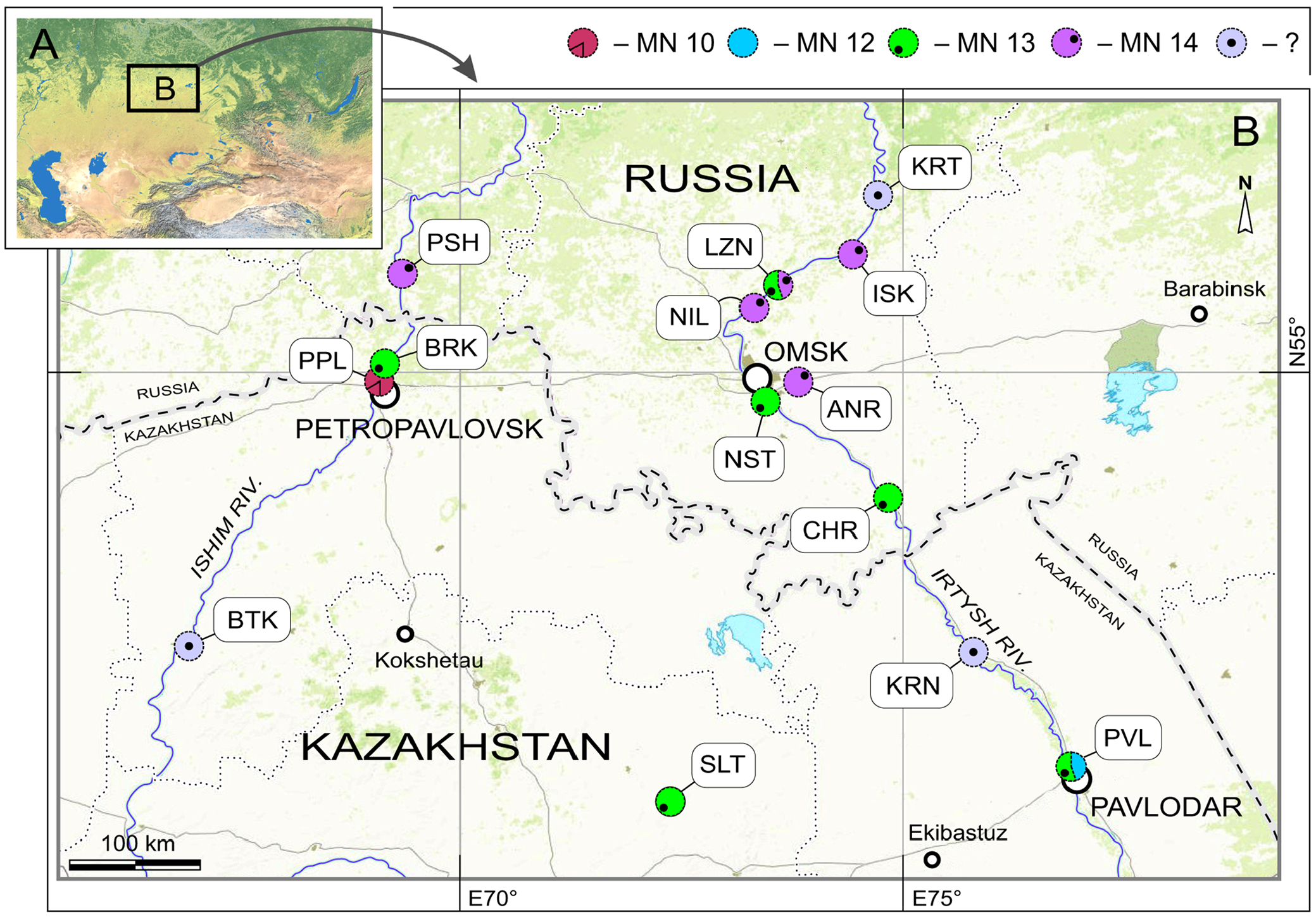
Type locality. Petropavlovsk GoogleMaps 1A, right slope of the Ishim River Valley, within Petropavlovsk town (ca. N54°54' E69°07'), North Kazakhstan Region, Kazakhstan ( Figure 1 View FIGURE 1 : PPL).
Type horizon. Stratotypic locality of Ishim Formation (ish), late Miocene (MN 10,?9.7–8.7 Ma). Material was collected by VZ during fieldworks of 1964, 1976, 1980, 1982 and 1987.
Type material. Holotype: GIN 952/1153 — right dentary fragment with p4–m1, the anterior alveolus of m2. Paratypes: (n = 7): GIN 952/1152 — left fragment of mandibular ramus with whole coronoid and condylar processes ; GIN 952/1154 — isolated left m2; GIN 952/1055 — isolated left M1; GIN 952/ 1158 — isolated right m1 ; GIN 952/1159 — isolated damaged crown part of the left i1 ; GIN 952/ 1160 — isolated right I1 with damaged root; GIN 952/1161 — isolated left I1.
Location of types. Holotype and paratypes in the collection of GIN, Moscow ( Russia).
Etymology. As for genus name, after the Ishim river.
Material. Type material and four remains from type locality (GIN 952/1157 — damaged right first lower molar; GIN 952/1400 — damaged left P4; GIN 952/ 1401 — damaged left P4; GIN 952/1402 — damaged left P4); and three rediposited remains (GIN 1115/1148 — worn left I1, crown part; GIN 1115/ 1149 — right i1; GIN 1115/1150 — left i1) from Borki 1A locality (Appendix 2: 1).
Measurements. See Appendix 4.
Diagnosis. As for genus.
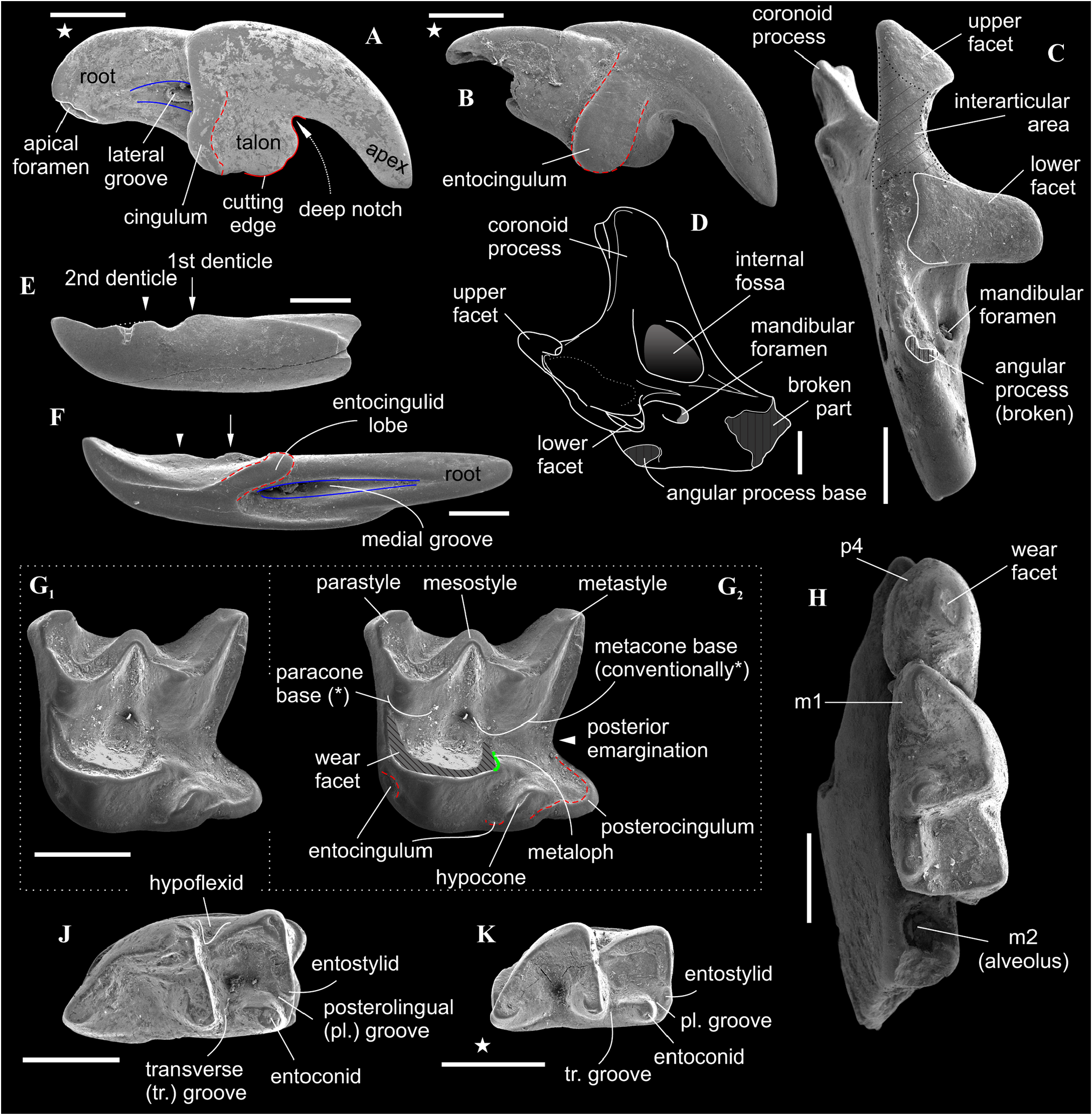
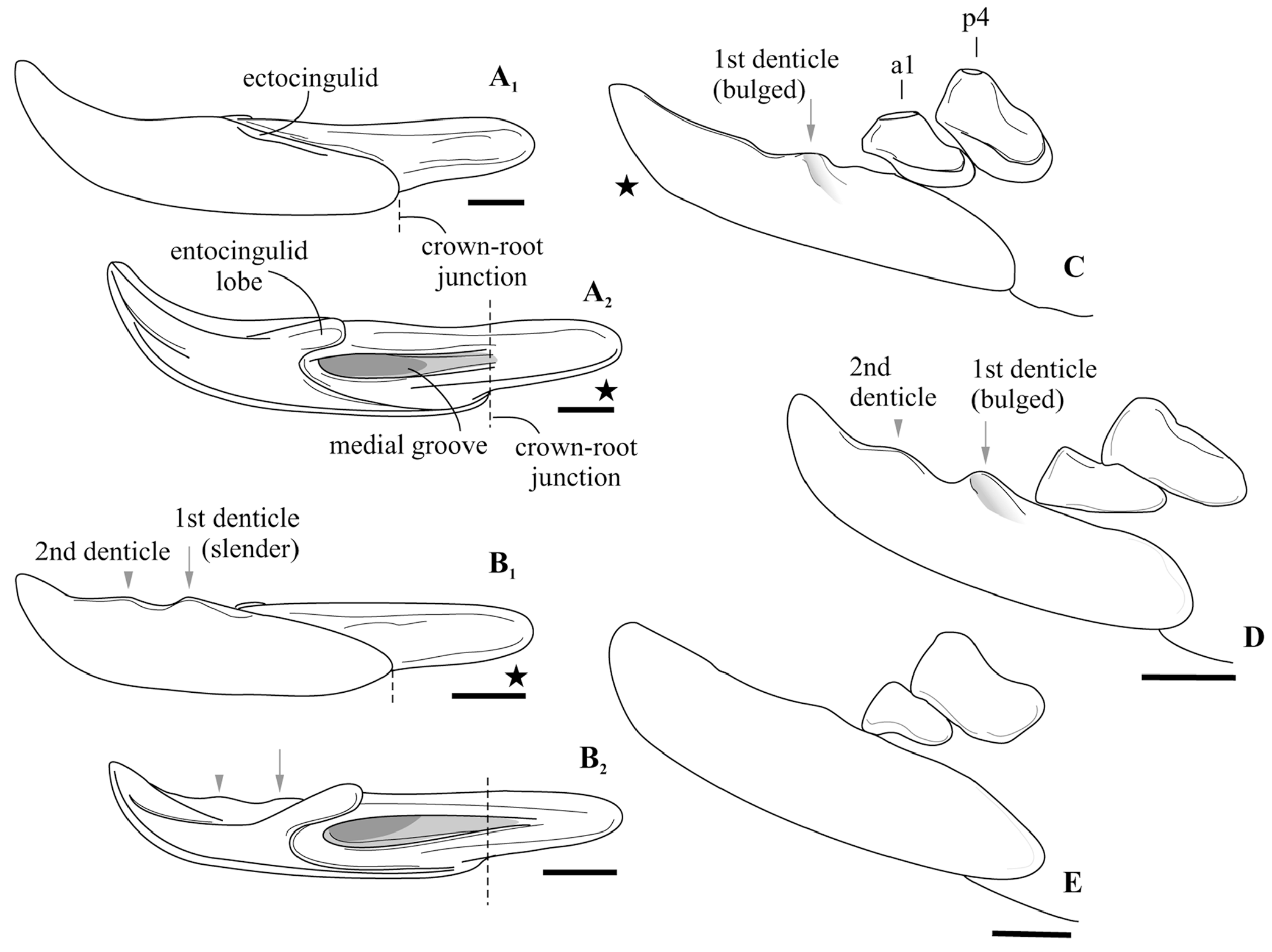
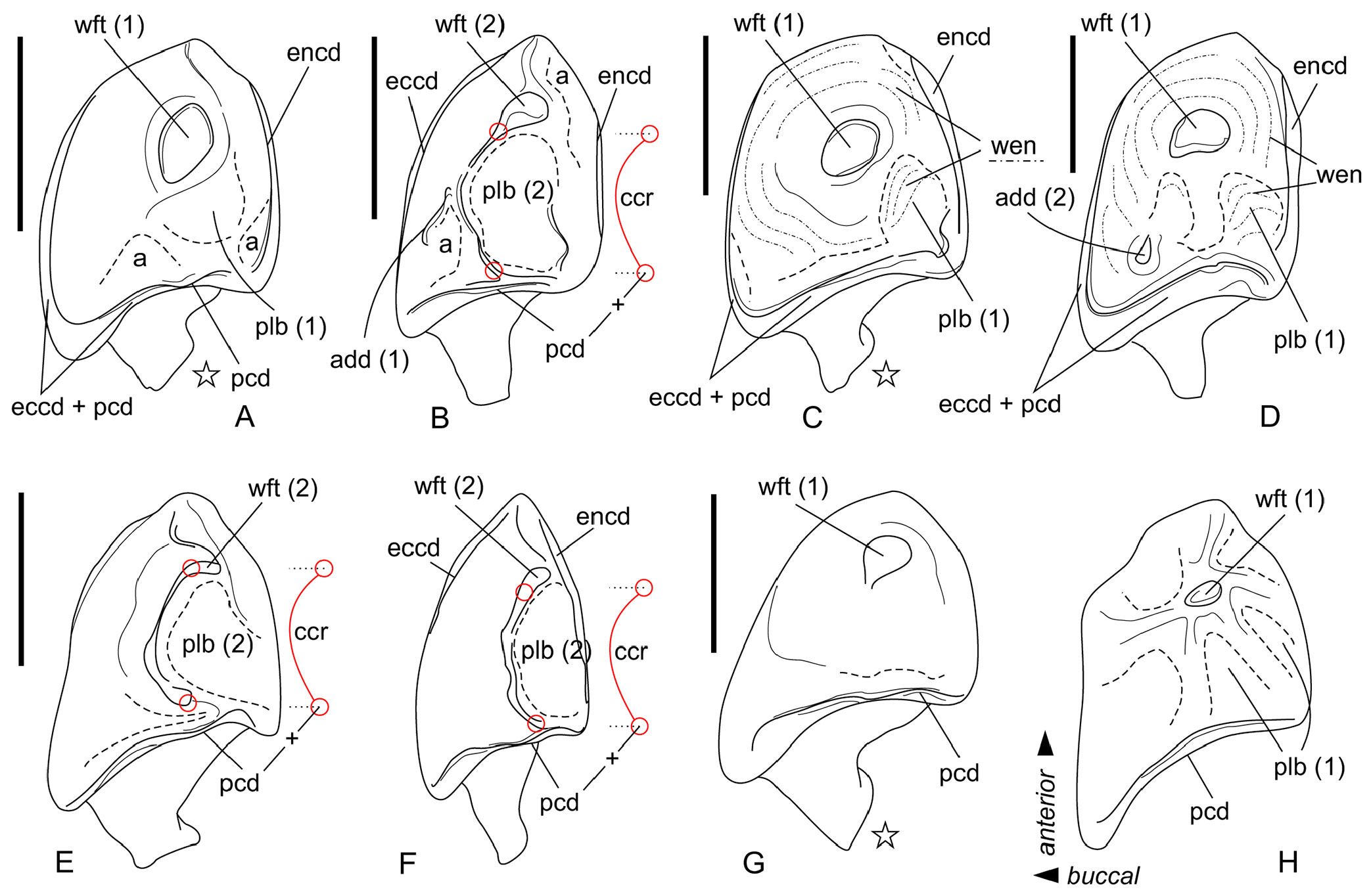
Description. I1 has an elongated massive crown; the large hatchet-like talon bears the sharp cutting edge and expressed narrow, deep notch; the apex of the incisor is not fissident. The buccal cingulum is wide and well expressed without a bulge and reaches half of the crown height. The root is notably shorter and more slender than the crown ( Figure 3A, B View FIGURE 3 ). Only three damaged fragments of the left P4 without lingual part are known. The buccal part of these teeth has a relatively short postparacrista; the small parastyle is weakly separated from the paracone base. M1 has a subquadrate occlusal shape with an approximately equal length of the lingual and buccal sides (BL/LL ratio; see Appendix 4); the mesostyle is well-developed and buccally protruded outward of the M1 base level; the buccal crests (preparacrista + ectoloph + postmetacrista) are represented as an expressed W-shaped line; the metaloph is short and separated from the metacone base; the hypocone is rounded distinct cusp and shifted to the lingual margin of the tooth; the weak ridge lays across the hypocone tip obliquely. The posterior emargination of M1 is relatively deep; the hypoconal flange is narrow and elongated backward ( Figure 2G View FIGURE 2 ). The relative distance between the hypocone and the posterior margin of the flange is distinctly larger than the other taxa have (see comparisons below). The crown of i1 is two times longer than the root (along the lateral side). The medial groove of the root is long and overreaches the crown-root junction. The cutting edge of i1 is bicuspidate; the basal (first) cuspule is slender without any bulge-like expression (opposite state to Crusafontina ); the distal cuspule is also weak expressed; an ectocingulid is absent ( Figure 2E, F View FIGURE 2 ). p4 has a massive and rounded crown; the central (single) wear facet is a rounded or ‘spot-shaped’ (opposite a ‘commashaped’ in Crisafontina; see van Dam, 2004: 744). The ecto- and entocingulids are well developed; the ectocindulid continuously passed to postcingulid ( Figure 4A View FIGURE 4 ). p4 bears a shallow posterolingual basin and extremely weak the central crest, which doesn’t reach the postcingulid. The lower molars are graded in size: the first molar is the largest, the second is moderately smaller than the first ( Figure 2H–K View FIGURE 2 ); the third molar is unknown. The hypoconid of m1 and m2 is protruded buccally and overhangs the base of the crown; the entostylid is presented as the small distinct bulge and does not reach the entoconid level. The entocristid is short and steeply descends to the metaconid base. The talonid basin opens posterolingually between the entoconid and entostylid through the posterolateral groove and lingually between the end of the entocristid and the metaconid base through the transverse groove. The narrow and well-distinguished ectocingulid does not reach the anterior side of the tooth ( Figure 2J, K View FIGURE 2 ). The buccal side of m1 has a slightly wrinkled enamel surface. The ectocingulid of m2 is well developed along the tooth base with the plate-like extension in the first third (paraconid level).
The horizontal ramus of the lower jaw is narrow; the small mental foramen is situated slightly backwards of the m1 protoconid level without groove. The mandibular ramus is relatively low; its anterior border is notably tilted backwards. The internal fossa of the temporal muscle ('internal fossa') is moderately developed. The condylar process bears the widely divided upper and lower articular facets and a moderately broad interarticular area ( Figure 2C, D View FIGURE 2 ).
Association of fragments. The studied remains from PPL/1A were each matched using the size recovery approach and several RSTs, A. squamipes and two Paranourosorex samples (Appendix 5: tables 5.1 and 5.2) for the following pairs of dental and mandibular elements and measurements: m1/ M1: measurements L(m1)/BL(M1); m1/I1: measurements L(m1)/H(I1); I1/i1: measurements L(I1)/ L(i1); m1/mandibular ramus: measuremets L(m1)/ COR and L(m1)/MRWc. Most of the matched pairs displayed compliance; e.g., the matched m1 and M1 corresponded to each other in size based on the RST ratio. Noncompliance (pink blocks in Table 5.2 of Appendix 5) was revealed for matched upper and lower incisors from PPL/1A (and BRK/1A), i.e., the observed dimension of the lower incisor was less than the supposed dimension calculated based on the Anourosorex ratio (Appendix 5: Table 5.1). This noncompliance between calculated and observed incisor sizes could be a specific feature of the particular species, which can be explained in terms allometric heterochrony (sense Mitteroecker et al., 2005: 250). This feature (the ratio between upper and lower incisors) in Ishimosorex ishimiensis gen. et sp. nov. differs from Anourosorex . In addition, the short lower incisor of Ishimosorex ishimiensis gen. et sp. nov. can probably be compensated through a relatively long mandible protraction during a chewing cycle (for details, see below remark on a phenomenon of ‘cutting edges straightening’). Proof of this is the best value for the ratio L(m1)/L(i1); i.e., the Anourosorex ratio for L(m1)/L(i1) corresponds more to the observed ratio in Ishimosorex ishimiensis gen. et sp. nov. than to the ratio L(I1)/L(i1). Thus, the studied remains from PPL/1A can be treated here as belonging to Ishimosorex ishimiensis gen. et sp. nov. based on metric matching (Appendix 5: Table 5.2).
Comparisons. Ishimosorex ishimiensis gen. et sp. nov. (L(p4) = 1.75 mm; L(m1) = 2.47–2.73 mm, see Appendix 4) differs in larger size from C. endemica (L(m1) = 1.90–2.12 mm, see van Dam, 2004: table 4), Crusafontina exculta ( Mayr and Fahlbusch, 1975) (L(m1) = 1.81–2.21 mm, see Prieto and van Dam, 2012), Crusafontina fastigata van Dam, 2004 (L(p4) = 1.43–1.44 mm, ibid.) and Crusafontina minima (Hutchinson and Bown, 1980) (L(m1) = 1.77–1.93 mm, see Bown, 1980: table VI). Ishimosorex ishimiensis gen. et sp. nov. differs from Crusafontina vandeweerdi van Dam, 2004 (L(p4) = 2.24 mm, van Dam, 2004: table 4) in its smaller size. Ishimosorex ishimiensis gen. et sp. nov. shows the similar teeth size to Crusafontina kormosi (Bachmayer and Wilson, 1970) (L(m1) = 2.38–2.84 mm, see Mészáros, 1998: table 3) and Crusafontina magna (Hutchinson and Bown, 1980) (L(m1) = 2.30–2.52 mm, Hutchinson and Bown, 1980: table V) but differs from both species in the well-developed mesostyle of M1, which contrary to C. kormosi and C. magna shows buccal protrusion of parastyle and metastyle tips (this is also a general difference from Anourosorex spp. ); in the extremely weak central crest and the spot-shaped wear facet of the p4 opposite well-developed central crest and comma-shaped wear facet of the p4 of C. kormosi ( Figure 4 View FIGURE 4 ); p4 of C. magna is unknown.
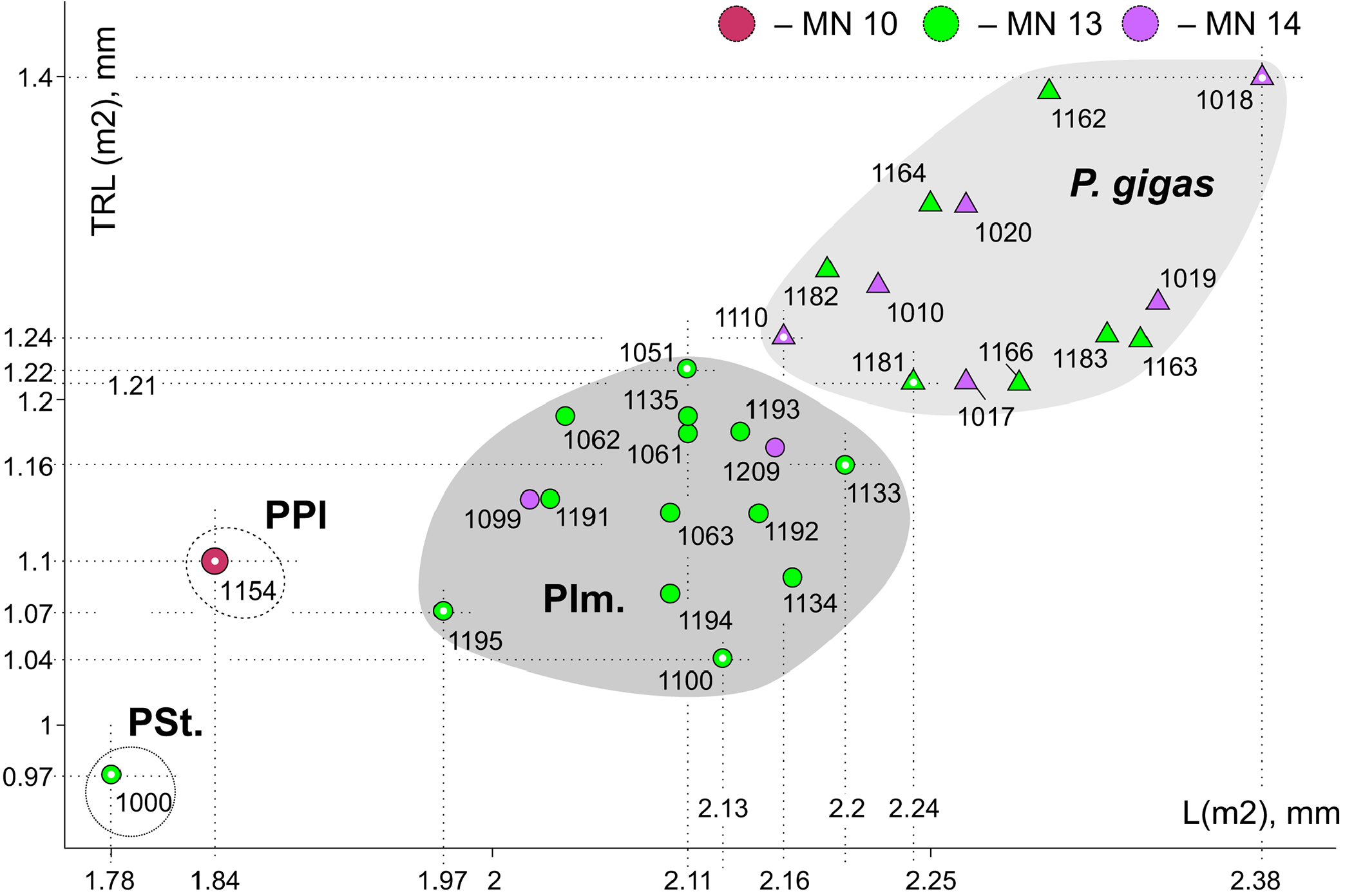
Ishimosorex ishimiensis gen. et sp. nov. differs from P. seletiensis in a slightly larger size; from Paranourosorex intermedius sp. nov. and P. gigas in a distinctly smaller size ( Figure 5 View FIGURE 5 ). Ishimosorex ishimiensis gen. et sp. nov. differs from P. inexspectatus in a slightly smaller size (Appendix 4). In addition, Ishimosorex ishimiensis gen. et sp. nov. morphometrically differs from the known Paranourosorex species in longer trigonid of m1 regarding the tooth length (TRL = 62.5% of m1 length) than show P. seletiensis (TRL = 57% of m1 length), Paranourosorex intermedius sp. nov. (TRL = 56% of m1 length) or P. gigas (TRL = 54% of m1 length).
The differences of Ishimosorex ishimiensis gen. et sp. nov. from species of Amblycoptus , Kordosia and Darocasorex were described in the new genus comparisons (see above the Differential Diagnosis section).
Remarks. Ishimosorex ishimiensis gen. et sp. nov. shows an intermediate combination of 'omnivorous' features of Paranourosorex and ‘carnivorous’ features of Crusafontina in the dentition. M1 bears expressed W-shaped buccal crests and a buccally protruding well-developed mesostyle similar to Paranourosorex conditions. In addition, both taxa have a moderately developed parastyle of M1, the bulbous and anteriorly inflated p4 without sharp crests and wide lower molars (buccal shifting of the protoconid and hypoconid). These features are intended for tearing and crushing, i.e., they can be determined as ‘omnivore-like features.’ In contrast, Crusafontina (American and European species, except C. vandeweerdi ), Amblycoptus , Kordosia and Anourosorex show different degrees of straightening of the buccal crests and increasing (from Crusafontina to Kordosia ) parastyle size of M1. The change in the parastyle together with the trapeziform M1 outline of these taxa probably are related to carnivorous adaptations such as the lengthening of cutting edges; e.g., to the sharp and well-developed central crest of p4 of American and European Crusafontina ; the central position of the protoconid of m1 and the corresponding lengthening of the preprotocristid of Crusafontina , Amblycoptus , Kordosia and Anourosorex to different degrees. Thus, some characteristics of Ishimosorex ishimiensis sp. nov. together with C. vandeweerdi can be determined as ‘carnivore-like’ compared with pronounced carnivorous anourosoricins. The deep notch in the talon of I1 and two sharp, slender denticles of the cutting edge of i1 are similar to the American and European species of the more carnivorous Crusafontina .
Stratigraphic and geographic range. Known from the type locality of the species (Petropavlovsk 1A, MN 10, Ishim Formation; North Kazakhstan Region) and Borki 1A locality (MN 13, Novaya Stanitsa Formation ; North Kazakhstan Region) to which the material was redeposited from Ishim Formation .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Soricomorpha |
|
Family |
|
|
SubFamily |
Soricinae |
|
Tribe |
Anourosoricini |
|
Genus |