Iraella ionescui, Pujade-Villar & Schiopu, 2015
|
publication ID |
https://doi.org/ 10.5252/z2015n3a5 |
|
publication LSID |
urn:lsid:zoobank.org:pub:41C2575F-B1B9-40E0-9140-1C06312B5AD8 |
|
persistent identifier |
https://treatment.plazi.org/id/038787CE-FFAF-3D6F-FC78-7D514D7C994C |
|
treatment provided by |
Felipe |
|
scientific name |
Iraella ionescui |
| status |
sp. nov. |
Iraella ionescui n. sp.
( Figs 1-3 View FIG View FIG View FIG ; 4D View FIG )
TYPE MATERIAL. — Holotype Comana-Amzacea ( Romania), 10.VI.2012: 1 ♀ (Schiopu col.) Ex Papaver rhoeas L. deposited in coll. JP-V.
Paratypes. (30♂♂, 35 ♀♀): Comorova Forest 44°2’12”N, 28°37’25”E (Constanta County, Dobrudja Province, Romania), Ex. Papaver rhoeas L., 15.VI.1996: 7 ♂♂, 7 ♀♀ GoogleMaps ; same data, 10.VI.1996: 2 ♂♂ GoogleMaps ; Comana-Pelinu 43°95’N, 28°4’E (Constanta County, Dobrudja Province , Romania) Ex. Papaver rhoeas L, 30.VI.1997: 17 ♂♂, 20 ♀♀ ; Constanta Port 44°14’N, 28°38’E (Constanta County, Dobrudja Province , Romania), Ex. Papaver rhoeas L, 04.VIII.2011: 3 ♂♂, 8 ♀♀ GoogleMaps ; Comana-Amzacea 43°54’N, 28°19’E (Constanta County, Dobrudja Province , Romania) Ex Papaver rhoeas L., 17.VI.2012: 1♂ GoogleMaps . Paratypes deposited in coll. JP-V. (15♂, 20 ♀), PHMBL (3 ♂, 3♀), USNM (3 ♂, 3 ♀), CAS (3 ♂, 3♀), AMNH (3 ♂, 3 ♀) and MNHN (3 ♂, 3 ♀).
TYPE LOCALITY. — Comana-Amzacea (Constanta county, Dobrudja province, Romania) situated at UTM coordinates 43°54’N, 28°19’E.
ETYMOLOGY. — This species is named in honour of Mihail Andrei Ionescu (1900-1988), a Romanian cynipidologist.
DIAGNOSIS. — The new species resembles I. hispanica , but differs in the following characters: mesoscutum strongly coriaceous (delicately coriaceous in I. hispanica ), mesopleuron reticulate (costulate-coriaceous in I. hispanica ), medial mesoscutal line present (inconspicuous or absent in I. hispanica ) and galls unilocular located at the base of stems (plurilocular in terminal flowers buds in I. hispanica ). The new species also resembles I. luteipes , but differs from the latter in having pronotal plate smooth and shiny (reticulate in I. luteipes ), F 1 in males similar to F2 (slightly modified in I. luteipes ), posterodorsal part of mesopleuron smooth and shiny (entirely and uniformly reticulate similar to the scutellum sculpture in I. luteipes ), elongated with scutellar foveae differentiated (confluent in I. luteipes ), radial cell around 2.5 times as long as wide (3.5 in I. luteipes ), stems with galls hypertrophied (unconspicous in I. luteipes ), and host plants being usually P. rhoeas and rarely P. dubium ( P. somniferum as in I. luteipes ).
DISTRIBUTION. — South-East Romania, including the Black Sea coast (Dobrudja province).
BIOLOGY. — Monovoltine species. Adults emerge in March-May when the host plant Papaver spp. is developing. Females lay eggs on poppy stems in April-May. Galls grow rapidly, so can be identified in the first half of May. Full development of galls ends in June-July. Larval stage last about 3-4 months and pupation occurs in October- November. Pupa development takes place in the larval chamber enclosed in a cocoon parchment. Overwintering takes place in gall as adult, whose development is completed sometimes in early December. The galls are synchronous with the other wasp galls from poppy capsules. Optimum time of collection: late June early July of development year. Most prospective parasitoids emerge from galls in June-July the first year and the rest next spring.
DESCRIPTION
Length
Females: 2.9-3.0 mm (n=7); males: 2.2-2.7 mm (n=8).
Coloration
Black; hypopygium, antennal flagellum and legs brown (femur, trochanter and coxa partially black).
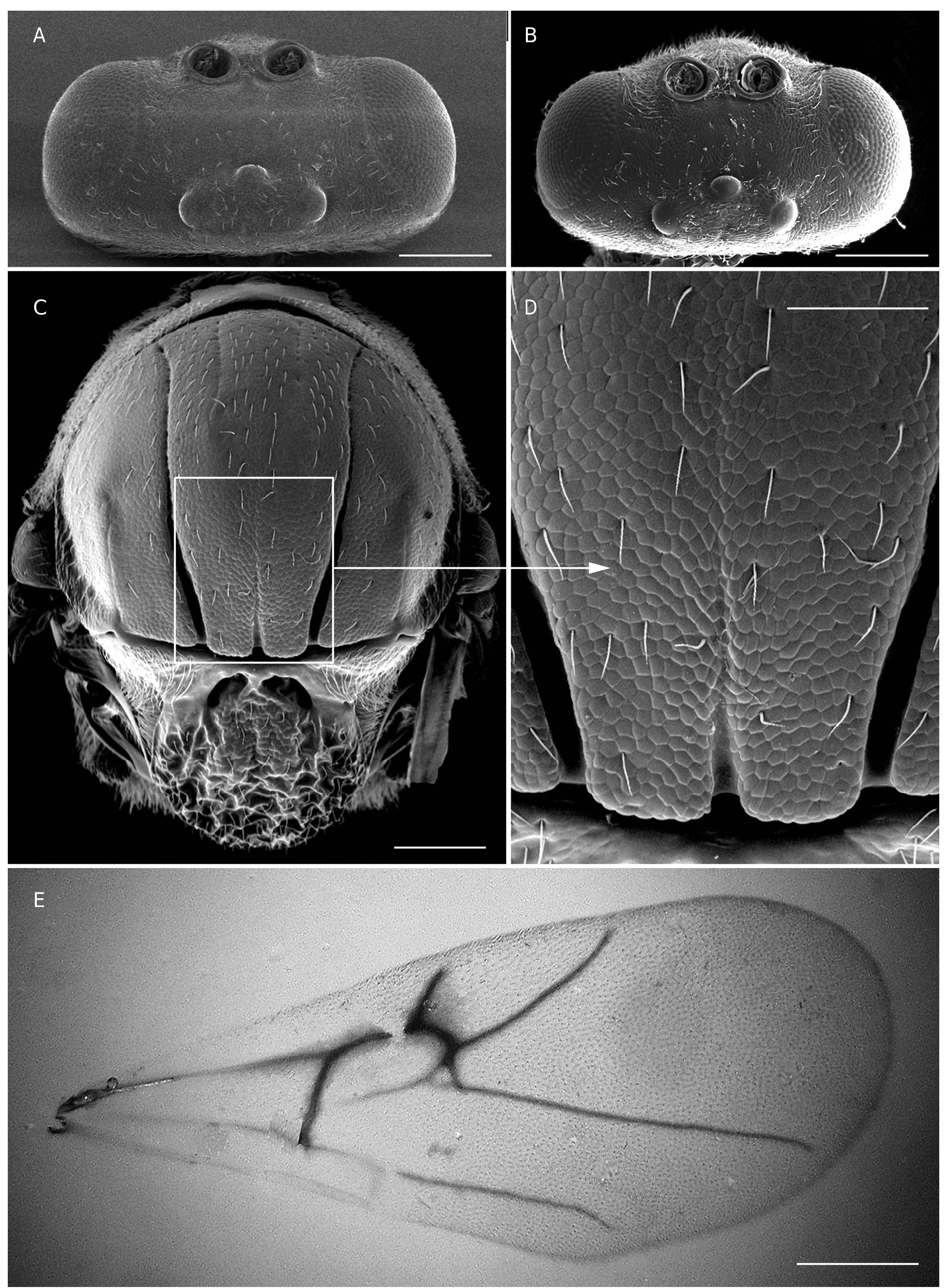
Head ( Figs 1A, B View FIG ; 2 View FIG A-C)
Head in dorsal view slightly more than twice as wide as long. In anterior view oval; 1.3 times as broad as high; lower face pubescent and with uniform coriarious sculpture; facial striae radiating from clypeus completely lacking. Upper face and vertex glabrous and more delicately sculptured than lower face. POL:OOL:OCO equal to 2.7:1.8:1.1 and related to ratio of diameter of lateral ocellus 0.8. Ocellar plate distinctly raised. Lateral margin of gena slightly curved, malar space around 0.45 times the height of compound eye. Clypeus more or less quadrangular, ventrally projecting over mandibles; ventral clypeal margin weakly incised; distance between anterior tentorial pits longer than the distance between epistomal sulcus and ventral margin of clypeus. Anterior tentorial pits conspicuous. Epistomal sulcus and clypeo-pleurostomal lines impressed, well-marked. Antennal toruli located at mid height of compound eye; distance between antennal rim and compound eye 0.8 times as wide as width of antennal socket including rim. Occiput pubescent, with rugulose sculpture, without occipital carina; some weak irregular transverse rugae present above occipital foramen.
Antennae
Female antenna ( Fig. 1 View FIG D-F) thin and elongate; 0.9 times as long as body, with 14 antennomeres; flagellum not broadening apically; placodeal sensilla present on all flagellomeres, weakly impressed; antennal formula: 5: 3 × 2.5: 7 × 2.5: 7: 6: 6: 6: 5: 5: 4.5: 4.5: 4: 4: 7. Male antenna ( Fig. 1 View FIG G-H) 1.1 times as long as body with 15 antennomeres; F1 not modified, staright; placodeal sensilla on all flagellomeres, weakly impressed; antennal formula: 5: 3 × 2.25: 7.5 × 2.25. 7: 6: 5: 5: 5: 5: 5. 5: 4.5: 4.5: 5.5: 5.
Mesosoma ( Figs 2C, D View FIG ; 3 View FIG A-D)
In lateral view convex dorsally, slightly longer than high. Dorsal pronotal margin concave in anterior view. Admedian pronotal depressions transversely oval, deep, open laterally, broadly separated medially by a distance as long as admedian depression length. Pronotal plate rectangular defined, hairless and without sculpture. Lateral surface of pronotum weakly coriarious; its dorsal and ventral parts with moderately long and dense pubescence. Mesoscutum 1.2 times as wide as long and 1.7 times as long as scutellum length; with strongly coriaceous sculpture; sparsely pubescent. Median mesoscutal impression distinct in posterior ⅓ of mesoscutum. Notauli complete; narrower and shallower anteriorly, broader, deeper and slightly convergent in posterior third of mesoscutum. Anterior parallel lines and parapsidal lines impressed. Mesoscutum and scutellum separated by a distinct transscutal suture. Scutellum rounded dorsally; strongly curved laterally, very slightly overhanging metanotum. Scutellar foveae ovate, deep, separated by a carina with posterior margins defined. Dorsal surface of scutellum strongly rugose. Axillula densely pubescent. Posterodorsal and posterior margins of axillula distinct. Posterior part of axillular strip extended dorsally. Mesopleuron uniformly reticulate without carinae, posterodorsal part of mesopleuron smooth and shiny, pubescent in lower half. Mesopleural triangle distinctly impressed and densely pubescent, with dorsal and ventral margins clearly marked. Metascutellum conspicuously constricted medially. Bar ventral to metanotal trough almost completely smooth; dorsal bar sculptured and pubescent. Metanotal trough narrow and densely pubescent. Metapleural sulcus slightly above of the mid-height of mesopleuron. Lateral propodeal carinae very weakly impressed, slightly divergent inferiorly. Lateral and median propodeal areas densely pubescent, smooth. Nucha relatively short; dorsally smooth.
Legs
Tarsal claws simple ( Fig. 1I View FIG ), without a basal lobe but broad basally, with a few long setae.
Forewing ( Fig. 2E View FIG )
Slightly longer than body, mostly hyaline, except slight infuscation around 2r, R1, R1 + S and M. Radial cell open along anterior margin, 2.5 times as long as wide. Rs and 2r curved. Areolet usually present. Cilia along apical margin short.
Metasoma
Female metasoma ( Fig. 3 View FIG E-G). In lateral view 1.4 times as long as high. Third abdominal tergum covering about half of metasoma, 3.2 times as long as fourth tergum; anteromedial area of third abdominal tergum with sparse setae but not forming a distinct patch. Fourth to seventh terga smooth, bare, with sparse micropunctures. Ventral spine of hypopygium with sparse micropunctures; projecting part short, longer than basal height of spine; ventrally with two double rows of short hairs.
Host plant
Papaver rhoeas L. rarely P. dubium L.
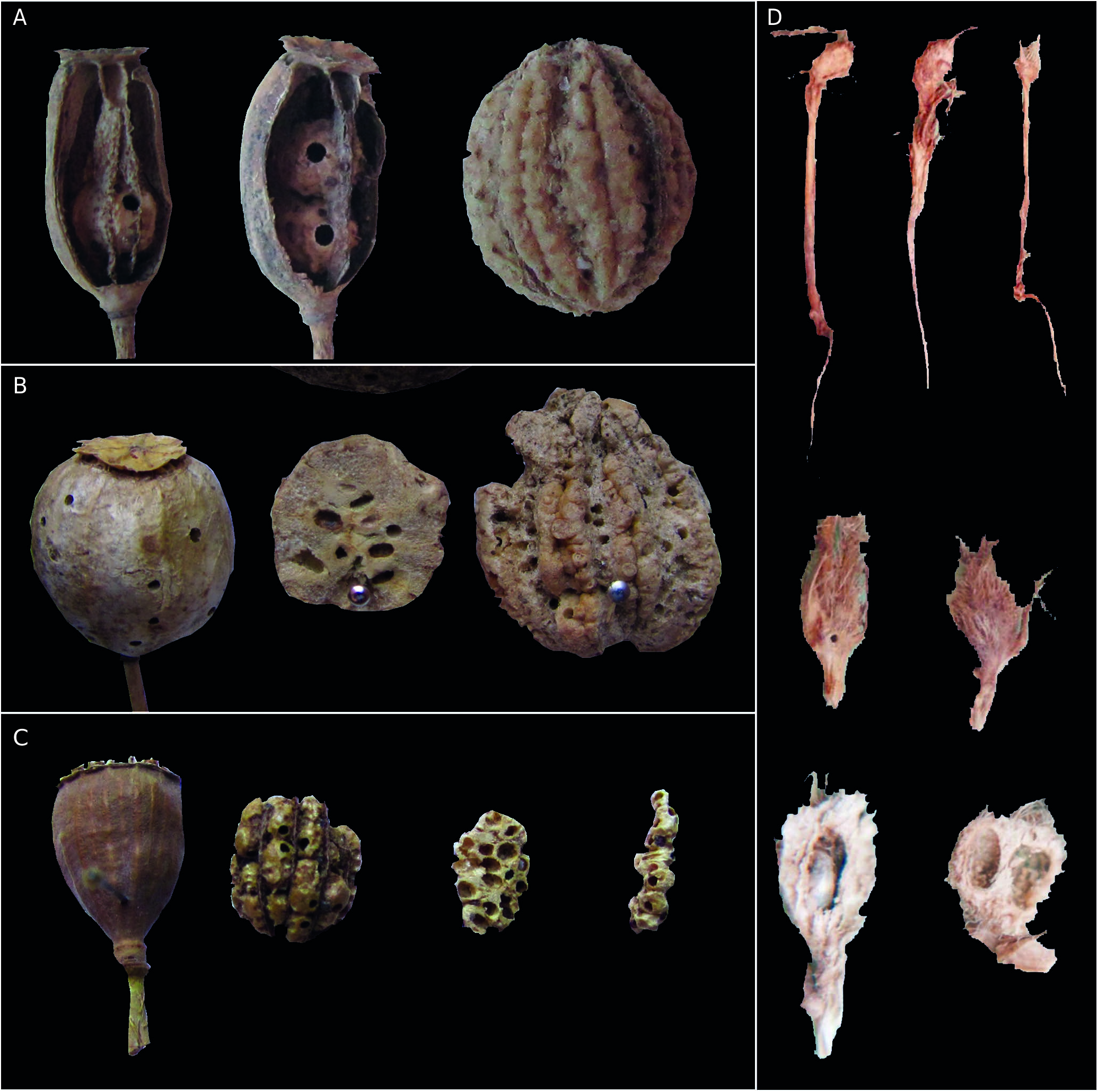
Gall ( Fig. 4D View FIG )
Unilocular galls are formed in the host plant stem at the base next to the roots ( Fig. 4D View FIG ) difficult to be seen and causing lateral or central deformation of the stem of puppies. The light green colored galls at early development will become yellow-brown as they mature. Most of the time galls are simple (monolocular), but bi- or trilocular galls may be formed by conglomeration of simple galls. Simple galls have an average diameter of approx. 4-5 mm and the multilocular galls about 10 mm. Host plant tissue with hypertrophy, galls are spherical or ovoid, covered with abundant silvery-white hair pilosity. Inside a gall was a simple larval chamber with no inside gall. In each larval chamber develops a single larva. Gall presence often inhibits the development of the host plant, giving it a stunt appearance. Sometimes plants with galls only reach a height of 4-5 cm. The galls are integral and they remain on the host plant until next spring when adults emerge.
COMMENTS
Some Aulacidea species as A. pilosellae (Kieffer, 1901) , A. scorzonerae (Giraud, 1859) and A. subterminalis (Niblett, 1946) , and Isocolus ponticus Dyakonchuk, 1982 , can produce galls in two different organs of host plant (leaves and midrib or flower head and stem). In all these cases the galls are similar and the adults identical. The species morphologically similar to I. ionescui n. sp. is I. hispanica , nevertheless, the galls of this new species are invariably unilocular and always found at the stem base whereas galls made by I. hispanica are plurilocular in terminal flowers buds. So far, no galls made by Iraela species, nor galls collected in puppies, have different morphology although these species have been abundantly collected in all European and circum-Mediterranean regions. This further support I. ionescui n. sp. as a new species, rather than being conspecific with I. hispanica support.
PARASITOIDS INDUCING GALLS ON PAPAVER
According to Askew et al. (2006) a total of 20 species of parasitoids are associated with the cynipid galls on Papaver ( Table 1), one species belongs to Figitidae ( Parnips nigripes ) while the rest are Chalcidoidea.
Parnips nigripes was originally described as a gall wasp in the Aulacidea View in CoL genus ( Barbotin 1964). Although Ronquist (1994) considered this species to be a figitoid inquiline, Ronquist & Nieves-Aldrey (2001) concluded that Aulacidea nigripes is neither a gall inducer nor a phytophagous inquiline; rather it is a koinobiont parasitoid of Barbotinia oraniensis . Based on its phylogenetic position, Ronquist & Nieves-Aldrey 2001 established a new genus and subfamily ( Parnipinae : Parnips ) for the species. Nonetheless, these authors did not designate the lectotype in spite of having examined the syntypes in the Barbotin collection.Therefore, we herein designate the lectotype (♀), which is deposited in coll. JP-V and bears the following labels: “ Oran Pap. rhoeas View in CoL , iii-60” (white label), “ Lectotype of Aulacidea nigripes Barbotin, 1964 , design. JP-V 2013” (red label), “ Parnips nigripes (Barbotin) JP-V det.” (white label). The paralectotypes are also deposited in JP-V collection (24♂, 40 ♀) and bear similar labels as the Lectotype (Assi Bou Nif, III.62: 1 ♂, 1 ♀; Murdjadjo, III.61 & III.62: 14 ♂, 20 ♀; Manguin, III.62: 1 ♂, 2 ♀; Moulay Ismael, III.62: 1 ♂, 1 ♀; Misserghin, III-IV.62: 1 ♂, 10 ♀; Oran, III-60 & II-61: III.62: 1 ♂, 6 ♀.
Nieves-Aldrey (2005) also obtained material of Parnips from Iraella hispanica and suggested that those specimens belong to a new species of Parnips based on a molecular analysis. Since no morphological difference was observed in comparison to P. nigripes (Nieves-Aldrey 2005) and he proposed that the specimens obtained from I. hispanica could be a sibling species of P. nigripes . We have some specimens of Parnips from Iraella ionescui n. sp. galls, and after examining tens of specimens, also did not found any morphological difference between these specimens and P. nigripes .
On Papaver galls, the most species of Chalcidoidea obtained are new for the Romanian fauna ( Table 1); only two species were known previously ( Andriescu 1971): Eurytoma infracta Mayr, 1904 and Chalcimerus borceai Stefan & Andriescu, 1962 , both on Barbotinia oraniensis . Four species are new for the Romanian fauna: Aprostocetus forsteri (Walker, 1847) and Baryscapus papaveris Graham, 1991 (Eulophidae) , Eupelmus aseculatus Kañlina, 1981 and E.atropurpureus Dalman,1820 (Eupelmidae) , according to Noyes (2012). The material was determined by Andriescu during 1994-2000; unfortunately some species were only determined to genus level: Aprostocetus sp. not belonging to A. forsteri , Eurytoma sp. not belonging to Eurytoma infracta and similar to Eurytoma robusta Mayr, 1878 , Mesopolobus sp. and Torymus sp. , which have never been cited in these galls, Ormyrus sp. not belonging to O. papaveris (probably belonging to O. capsalis Askew, 1994 ), Pteromalus sp. (probably belonging to P. hieracii (Thomson, 1878) or P. papaveris Förster, 1841 ). The other twelve species are determined to species ( Table 1).
In the new species, Iraella ionescui n. sp., the parasitoids obtained are: Parnips nigripes , Eupelmus atropurpureus , E. vesicularis (Retzius, 1783) (sensu Fusu 2010), Eurytoma robusta , Idiomacromerus mayri (Wachtl, 1883) , Mesopolobus sp. and Trichomalus tenellus (Walker, 1834) . These results contrast with I. hispanica where chacidoids have never been obtained.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Iraella ionescui
| Pujade-Villar, Juli & Schiopu, Ion 2015 |
ionescui
| Pujade-Villar & Schiopu 2015 |
Iraella ionescui
| Pujade-Villar & Schiopu 2015 |
Iraella hispanica
| Nieves-Aldrey 2005 |
Iraella hispanica
| Nieves-Aldrey 2005 |
I. hispanica
| Nieves-Aldrey 2005 |
Parnips
| Ronquist & Nieves-Aldrey 2001 |
Parnips
| Ronquist & Nieves-Aldrey 2001 |
Parnips
| Ronquist & Nieves-Aldrey 2001 |
Parnips
| Ronquist & Nieves-Aldrey 2001 |
Barbotinia
| Nieves-Aldrey 1994 |
Iraella
| Nieves-Aldrey 1994 |
Ormyrus capsalis
| Askew 1994 |
Baryscapus papaveris
| * Graham 1991 |
Eupelmus aseculatus * (Kañlina, 1981)
| * (Kanlina 1981 |
Aulacidea nigripes
| Barbotin 1964 |
Aulacidea nigripes
| Barbotin 1964 |
Chalcimerus borceai Steffan &
| Steffan & Andriescu 1962 |
Eurytoma infracta
| Mayr 1904 |
Aylacini
| Ashmead 1903 |
Aulacidea
| Ashmead 1897 |
Eurytoma robusta
| Mayr 1878 |
Figitidae
| Thomson 1862 |
Eupelmus microzonus FÖrster, 1860
| Foerster 1860 |
Ormyridae FÖrster, 1856
| Forster 1856 |
Glyphomerus tibialis FÖrster, 1856
| Forster 1856 |
Aprostocetus forsteri
| * (Walker 1847 |
Pteromalus papaveris FÖrster, 1841
| Forster 1841 |
Aylax
| Hartig 1840 |
Aylax minor
| Hartig 1840 |
Eurytoma cynipsea
| Boheman 1836 |
Eupelmidae
| Walker 1833 |
Torymidae
| Walker 1833 |
Eurytomidae
| Walker 1832 |
Sycophila mellea
| Curtis 1831 |
Eulophidae
| Westwood 1829 |
Eupelmus atropurpureus
| * Dalman 1820 |
Pteromalidae
| Dalman 1820 |