Hyalinobatrachium anachoretus, Twomey, Evan, Delia, Jesse & Castroviejo-Fisher, Santiago, 2014
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3851.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:9840D64B-F08C-44E7-B2DC-4818F8FFDD4F |
|
DOI |
https://doi.org/10.5281/zenodo.6136429 |
|
persistent identifier |
https://treatment.plazi.org/id/664887B1-FFFF-FF95-FF7C-FDCDD659F887 |
|
treatment provided by |
Plazi |
|
scientific name |
Hyalinobatrachium anachoretus |
| status |
sp. nov. |
Hyalinobatrachium anachoretus View in CoL new species
Figures 24 View FIGURE 24 , 25 View FIGURE 25
Holotype. CORBIDI 10472, adult male collected from a stream on the east slope of Abra Patricia (the divide between the Río Marañón and Río Huallaga drainages), 2.2 km by road NE from Puente Nieva, Provincia Rioja, Departamento San Martín, Peru, 5°40'39.06"S, 77°46'23.99"W, 2001 m, collected by E. Twomey, T. Kosch, and M. Panaijo on 5 May 2011.
Paratopotype. CORBIDI 10462, adult male, same locality and collection data as holotype.
Generic placement. The new species is placed in the genus Hyalinobatrachium on the basis of our phylogenetic analyses that included complete or partial sequences of the mitochondrial genes 12S and 16S. Additionally, the following phenotypic characters typical of Hyalinobatrachium ( Guayasamin et al. 2009) are present in the new species: (1) humeral spine absent in adult males; (2) digestive tract and bulbous liver covered by iridophores (white); (3) ventral parietal peritoneum and pericardium completely transparent; (4) white bones in life; (5) when present, nuptial pads small and restricted to the medial edge of Finger I in males; (6) dentigerous process of the vomer and vomerine teeth absent; (7) males seen vocalizing from the undersides of leaves; and (8) eggs deposited on the undersides of leaves. However, the value of these characters as synapomorphies for Hyalinobatrachium needs to be tested.
Diagnosis. The following combination of characters can distinguish Hyalinobatrachium anachoretus from other species in the family: (1) dentigerous process of the vomer and vomerine teeth absent; (2) snout truncate in dorsal and lateral view; (3) tympanum barely visible with equal coloration and granulation as surrounding skin; (4) dorsal skin weakly granular on body, slightly more granular on lateral regions of the head; (5) ventral skin granular, cloacal ornamentation absent, paired round tubercles below vent absent; (6) parietal peritoneum transparent; heart and urinary bladder lacking iridophores (= uncovered), kidney with iridophores on medial third, lateral two-thirds of kidney uncovered; liver and viscera mostly covered by iridophores except for inferior surface of liver and the adjacent surface of the intestine which are uncovered; testes completely covered by iridophores; (7) liver bulbous; (8) humeral spine absent in adult males; (9) finger webbing absent between fingers I and II, basal webbing between Fingers II and III, III 2 - 2 - IV; (10) toe webbing I 1 + - 2- II 1 + - 1+ III 1 - - 1+ IV 1 + - 1- V; (11) fringe on postaxial edge of finger IV present, metacarpal fold and ulnar fold present and enameled, metatarsal and tarsal fold present and enameled; (12) nuptial excrescence present as a small pad on medial edge of Finger I (Type V), prepollex not enlarged; prepollical spine not projecting (spine not exposed); (13) when appressed, finger I longer than II; (14) diameter of eye roughly 2.2 times wider than disc on Finger III; (15) coloration in life: dorsal surfaces apple green with small yellow spots and minute melanophores, bones white; (16) coloration in preservative pale yellow, dorsum scattered with dark melanophores; (17) iris coloration in life: creamy white with irregular dark flecks; flecks concentrated around pupil giving impression of diffuse grey ring; (18) distribution of melanophores on digits constant in both specimens, absent from fingers, present on toes IV and V; in life hands and feet are light green, tips of fingers and toes pale orange; (19) males were seen calling from the undersides of leaves; advertisement call consisting of a single note, distinctly pulsed (5–6 pulses); call duration note 0.32– 0.37 s, dominant frequency 4670–4800 Hz with no apparent frequency modulation; (20) clutches were located on the lower surfaces overhanging streams, clutch size 19– 20 eggs (N = 3); (21) SVL in males 20.6–21.4 mm (N = 2); (22) enameled glands absent from lower part of upper lip and skin covering jaw.
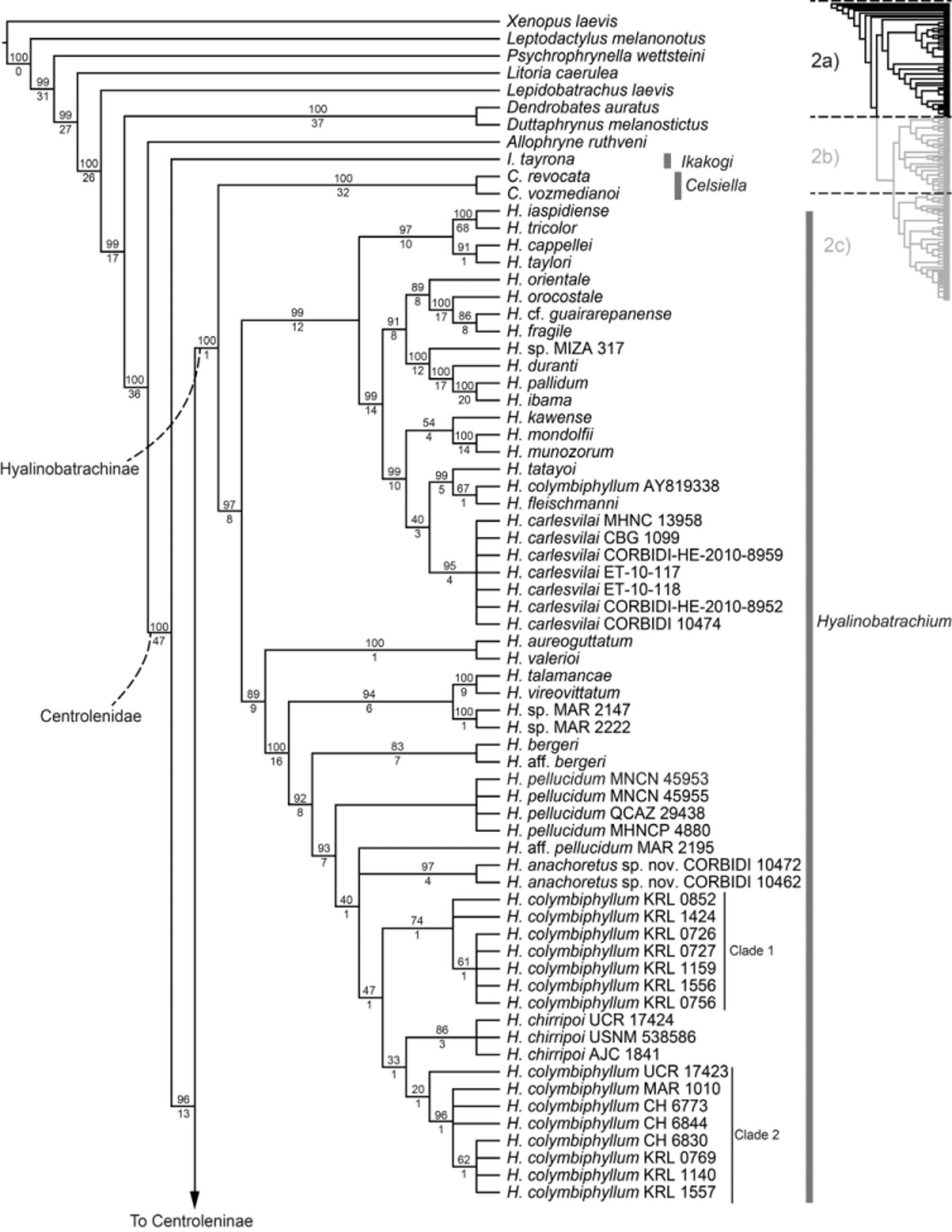
Comparisons. Many species of Hyalinobatrachium are difficult to diagnose using only morphological characters, especially when using preserved specimens (Castroviejo-Fisher et al. 2009; 2011). In our diagnosis we combine typical morphological characters (in most cases they show only subtle differences that might need a trained eye and direct comparisons of specimens to detect), with bioacoustic evidence and phylogenetic relationships inferred from DNA sequences (Table 3, Fig. 2a View FIGURE 2 a ). As the new species occurs in the east-Andean versant, we limit our comparisons to species which occur in this ecoregion, except for two closely related species occurring west of the Andes: H. colymbiphyllum and H. chirripoi , both of which occur in Central America and northeastern South America ( H. colymbiphyllum ranges into the Río Cauca and Río Magdalena valleys of Colombia). Character states of H. anachoretus are in parentheses. Hyalinobatrachium bergeri has a hand webbing formula III 3 - 2 + IV (III 2 - 2 – IV); advertisement call consisting of a single monotone note, call duration 0.129– 0.198 s (single trilled note with 5–6 pulses, 0.32– 0.37 s); pericardium with at least some iridophores (pericardium without iridophores); they are not sister species in phylogenetic analysis. Hyalinobatrachium carlesvilai has a hand webbing formula III 2 – - 1+ IV (III 2 - 2 – IV); advertisement call consisting of a single note, the first third being pulsed and the second third tonal, call duration 0.102– 0.152 s (single trilled note with 5–6 pulses, 0.32– 0.37 s); pericardium with at least some iridophores (pericardium without iridophores); they are not sister species in phylogenetic analysis. Hyalinobatrachium chirripoi has “extensive” hand webbing (as described by Kubicki 2004, 2007) (hand webbing moderate, i.e., III 2 - 2 – IV); advertisement call with pulse rate 55–57 pulses/s (13.8–19.2 pulses/s); SVL 23.7–26.4 mm (20.6–21.4 mm). Hyalinobatrachium colymbiphyllum has a single trilled note advertisement call with 7–9 pulses and lower dominant frequency 3375–3658 Hz (single trilled note with 5–6 pulses and higher dominant frequency 4670–4800 Hz); SVL 23.0– 30.6 mm (20.6–21.4 mm); yellow or gold iris in life (white or cream iris). Hyalinobatrachium esmeralda has a hand webbing formula III 2 + - 2+ IV (III 2 - 2 – IV); pericardium white or partially pigmented (transparent, but see Fig. 23 View FIGURE 23 ), single tonal note advertisement call (single trilled note with 5–6 pulses) (M. A. Rada, pers. comm.); snout rounded in dorsal and lateral views (truncate). Hyalinobatrachium ibama has a white pericardium (transparent); dorsal surfaces with minute and small melanophores (only minute melanophores); they are not sister species in phylogenetic analysis. Hyalinobatrachium mondolfii has a hand webbing formula III 2 - (1+ – 1 1/2) IV (III 2 - 2 – IV); single tonal note advertisement call (single trilled note with 5–6 pulses); pericardium white (transparent); snout rounded in dorsal and lateral views (truncate); they are not sister species in phylogenetic analysis. Hyalinobatrachium munozorum has a hand webbing formula III 2 – - 1+ IV (III 2 - 2 – IV); pericardium white (transparent); snout rounded in dorsal and lateral views (truncate); they are not sister species in phylogenetic analysis. Hyalinobatrachium pellucidum has a single tonal note advertisement call, each note 0.12– 0.15 s in duration, dominant frequency 4863–5408 Hz (vs. single trilled note with 5–6 distinct pulses, notes 0.32– 0.37 s in duration, dominant frequency 4670–4800 Hz); they are not sister species in phylogenetic analysis. Hyalinobatrachium ruedai has a hand webbing formula III 2 - 1 3/ 4 IV (III 2 - 2 – IV); pericardium white (transparent); snout rounded in dorsal and lateral views (truncate).
Description of the holotype. Adult male with SVL 21.4 mm. Head just wider than body; head width 38% of SVL; head width 1.32 times head length. Snout truncate in dorsal and lateral view; eye-nostril distance/eye diameter = 1.12 and eye-nostril distance/interorbital distance = 0.66. Loreal region flat and nearly vertical, nostrils slightly protuberant, elliptical; internarial region concave anterodorsally; canthus rostralis well defined. Eyes small, directed anterolaterally, eyes 47.5° relative to midline (where anterior-facing eyes would be 90° relative to midline); eye diameter 2.2 times wider than width of disc on finger III; eye diameter 36% of head length and 59% of interorbital distance. Tympanum hardly visible, with no annulus nor supratympanic fold visible externally, center colored as the dorsum; tympanum with slight dorsal inclination. Dentigerous processes on vomers absent, choanae large, circular, separated more widely than nostrils; tongue round, white in preservative, anterior 2/3 attached to mouth; vocal slits present, extending along floor of mouth lateral to tongue; enameled glands absent from lower part of upper jaw. Forelimbs slim, with forearm roughly 1.5 times as wide as arm; ulnar fold present and enameled; humeral spine absent. Relative length of fingers: I <II ≈ IV <III; finger discs wide, rounded, larger than toe discs, disc on finger III 45 % of eye width; finger webbing absent between fingers I and II, basal webbing between fingers II and III, webbing moderate between fingers III and IV with formula III 2 - 2 - IV. Prepollex concealed; subarticular tubercles round, faint; supernumerary tubercles not noted, palmar tubercle round and small, thenar tubercle ovoid; nuptial excrescences present as a small pad on medial edge of Finger I (Type V); faint crest running down lateral edge of hand from base of Finger IV to base of hand. Hind limbs slender, tibia length 54% of SVL; tarsal fold present and enameled; discs of toes small, round, inner metatarsal tubercle small, slightly conical; outer metatarsal tubercle not noted. Webbing formula of feet: I 1 + - 2- II 1 + - 1+ III 1 - - 1+ IV 1 + - 1- V. In preservative, dorsal skin peppered with small dark melanophores; dorsal skin weakly granular on body, slightly more granular on lateral regions of the head; skin on ventral side smooth, except on abdomen which is granular; cloacal opening at level of upper thighs, cloacal ornamentation present as an enameled cloacal fold. Parietal peritoneum transparent; heart and urinary bladder lacking iridophores; kidney with iridophores covering medial third, lateral two-thirds of kidney uncovered; liver and viscera mostly covered by iridophores except for inferior surface of liver and the adjacent surface of the intestine which are uncovered; gonads completely covered by iridophores. Kidneys rounded, approximately bean-shaped; liver bulbous.
Coloration in life. Overall impression is apple-green with irregular, diffuse yellow blotches. Dorsum peppered with small melanophores that appear dark green ( Fig. 24 View FIGURE 24 ). Legs and arms pale green, melanophores absent in circular patches giving the impression of faint pale-yellow spots. Finger and toe discs pale orange. Flanks white. Iris creamy white with irregular dark flecks. Flecks concentrated around pupil giving impression of diffuse grey ring surrounding pupil. Ventrally, parietal peritoneum transparent; pericardium also transparent with heart showing red in life; visceral peritoneum of gall bladder and urinary bladder also transparent. Hepatic and visceral peritonea white. Ventral vein red, prominent.
Coloration in preservative. General appearance pale yellow ( Fig. 25 View FIGURE 25 ). Dorsal surfaces dotted with sparse melanophores; faint yellow spots present on the legs. Venter appearing uniform white. Iris and peritonea as in life.
Measurements. Holotype measurements (in mm) with paratype measurements given in parentheses: SVL 21.4 (20.6), HL 6.2 (6.1), HW 8.2 (8.0), TD 0.8, IND 1.3 (1.5), IOD 3.4 (3.4), ED 2.0 (2.0), EW 3.0 (2.9), END 2.2 (2.3), HaL 6.0 (5.3), 3DW 0.9 (0.9), TL 11.2 (10.5), SL 11.6 (11.6), FL 9.1 (8.9). Note that in the paratype, the tympanum was not visible, thus tympanum diameter is not reported.
Variation. In life, the holotype has slightly more prominent spots on the legs than the paratype. One uncollected male, which was photographed near an egg clutch ( Fig. 24 View FIGURE 24 ) had more prominent spotting on the dorsum than either of the types. In the paratype, iridophores cover just the medial-most edge of the kidneys, whereas the inner third of the kidneys have iridophores in the holotype.
Vocalizations. Numerous males were calling on the night the type series was collected. The analysis here is based on a recording of a single male from the type locality, taken at 15.5°C. Although the recorded call belongs to an uncollected male, we (ET) observed a male calling from the underside of a leaf next to eggs, so the call reported here is definitely attributable to H. anachoretus . The entire recording is 58 s long and contains 4 notes all given by the same male. The call consists of a single note that is distinctly pulsed, with notes given 13–16 s apart. Each note is 0.32– 0.37 s in duration, containing 5–6 distinct pulses (pulse rate 13.8–19.2 pulses/s). Dominant frequency 4670–4800 Hz with no apparent frequency modulation. Unfortunately, the recording has too much background noise for illustration purposes; however, the recording is of high enough quality to extract relevant call parameters.
This pulsed call is unlike any call known from Hyalinobatrachium in the east-Andean versant, and is most similar to H. colymbiphyllum , which occurs in southeastern Central America and the Pacific lowlands and Río Cauca and Magdalena drainages of Colombia ( AmphibiaWeb, 2014). However, there appear to be some differences in calls between these species. We (JD) have recorded 5 individuals of H. colymbiphyllum from Río Frijoles ca. 300 m, Parque Nacional Soberanía, Panama, and the calls have more pulses (7–9 pulses in H. colymbiphyllum vs. 5–6 in H. anachoretus ), and lower dominant frequency (3375–3658 Hz in H. colymbiphyllum vs. 4670–4800 Hz in H. anachoretus ). Hyalinobatrachium chirripoi and H. pellucidum (two other closely related species to H. anachoretus ) have distinct calls. The call of H. chirripoi (based on a recording from a single male found along a tributary of Río Banano at 80 m, Limón, Costa Rica; Kubicki 2004, call courtesy B. Kubicki) is much more highly pulsed (pulse rate 55–57 pulses/s in H. chirripoi vs. 13.8–19.2 pulses/s in H. anachoretus ), whereas the call of H. pellucidum is a single, monotone, non-pulsed note ( Wen et al. 2012). The advertisement calls of Hyalinobatrachium esmeralda and H. aff. pellucidum have not been described; however, we have analyzed recordings of each taxon (from the type locality and from Chivor, Boyacá, respectively) graciously provided by M. A. Rada. A full description of the calls will be published elsewhere (M. A. Rada in prep.), but both are clearly different from that of H. anachoretus in that they are composed of a single, unpulsed, tonal note.
Distribution and ecology. Hyalinobatrachium anachoretus is known only from the vicinity of the type locality ( Fig. 18 View FIGURE 18 ). Males were also heard calling in a stream near Puente Nieva, which is roughly 2 km by road from the type locality and located at 2050 m. As far as we can tell, the latter site is also the type locality of Centrolene lemniscatum and Ce. muelleri ( Duellman & Schulte 1993), although we have not encountered either species at that site. Thus, H. anachoretus appears to be sympatric with Ce. lemniscatum and Ce. muelleri at least in this area.
Calling behavior and general activity in this species is perplexing. We have visited the type locality at night on three occasions (24 May 2010, 5 May 2011, and 14 June 2011) and were only able to hear and find frogs on the night of 5 May 2011. In addition, R. Schulte visited this site at least once while collecting glassfrogs (the types of Ce. lemniscatum and Ce. muelleri were collected 9 July 1989 from a stream less than 2 km from the type locality of H. anachoretus , a site where we also heard H. anachoretus calling on 5 May 2011), yet H. anachoretus was not recorded. It is worth mentioning that on the one night these frogs were active they were abundant and conspicuous, with high densities of males calling along every drainage in the area (even Río Nieva, the largest drainage in the area). Weather conditions are known to affect calling rates in some Central American Hyalinobatrachium , with a decline in vocal activity during drier evenings (Vockenhuber et al. 2008; Kubicki 2007). During the three nights when we visited the area there was significant rainfall and high humidity, typical for this high cloud forest region. A possible explanation is that the breeding season of H. anachoretus ends sometime in early to mid-May, which coincides with the end of the wet season.
Although we did not directly observe oviposition, three clutches were found near calling males ( Fig. 24 View FIGURE 24 ). These were typical of Hyalinobatrachium , consisting of a monolayer array of unpigmented eggs attached to the lower surfaces of broadleaf vegetation. Clutch sized ranged from 21– 25 eggs (N = 3), which is notably smaller than that of H. chirripoi and H. colymbiphyllum . Hyalinobatrachium chirripoi clutch size ranges from 65– 80 eggs ( Kubicki 2004). In H. colymbiphyllum from near Gamboa, Panama clutch sized ranged from 51– 102 eggs (JD, unpublished), and near Rincón de Osa, Costa Rica, clutch size ranged from 33– 70 eggs ( McDiarmid 1978). Kubicki (2007) reported smaller clutches (25– 35 eggs) in a high-elevation population near Monteverde, Costa Rica. In the closely related H. pellucidum clutch size ranged from 24– 41 eggs near San Jose (JD, unpublished.). Combat behavior, tadpoles, and parental care are unknown for this species.
Etymology. The name is derived from the Latin noun anachoretus , which means ‘hermit’. This name seems appropriate for two reasons. First, this species appears to be isolated from other members of the genus in that it occurs in the cool, rainy highlands of the Alto Mayo drainage. Second, the species seems to be reclusive, as we have only been able to locate it on a single night of collecting despite several visits to the locality.
Remarks. Our phylogeny shows H. anachoretus as part of a clade containing H. aff. pellucidum , H. colymbiphyllum , and H. chirripoi . The topology we recovered did not support a monophyletic H. colymbiphyllum . One interpretation of this topology would be that H. chirripoi is a synonym of H. colymbiphyllum . However, H. chirripoi has a distinct advertisement call (see above) and is diagnosable from H. colymbiphyllum on the basis of hand webbing (extensive in H. chirripoi vs. moderate in H. colymbiphyllum ; Kubicki 2004). Another possibility is that one of the clades of H. colymbiphyllum represents an undescribed cryptic species. If this is the case, the two species would be sympatric in El Cope, Panama (i.e., all terminals in clade 1 are from El Cope; in clade 2, terminals KRL 0 769, KRL 1140, KRL 1557 are also from El Cope). It is worth mentioning that Crawford et al. (2010), the study for which many of these sequences were generated, also recovered two distinct clades of H. colymbiphyllum . However, H. chirripoi was not included in their analysis. Based on these results, we strongly encourage further investigations of a possible cryptic species of Hyalinobatrachium in El Cope, Panama.
| CORBIDI |
Centro de Ornitologia y Biodiversidad |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |