Hubrechtella ijimai ( Takakura, 1922 ) Kajihara, 2006
|
publication ID |
https://doi.org/ 10.5281/zenodo.2645302 |
|
persistent identifier |
https://treatment.plazi.org/id/03825157-FF93-5642-FB3F-91EEFC3AFAF4 |
|
treatment provided by |
Plazi |
|
scientific name |
Hubrechtella ijimai ( Takakura, 1922 ) |
| status |
comb. nov. |
Hubrechtella ijimai ( Takakura, 1922) comb. nov. ( Figs 15–19 View FIGURE 15 View FIGURE 16 View FIGURE 17 View FIGURE 18 View FIGURE 19 )
Diagnosis
Hubrechtella with a long ‘tail’; bodywall musculature without zigzag fibres; probos cis musculature with inner circular, middle longitudinal, and outer circular layers, with single muscle cross; proboscis epithelium with spherical bodies; middorsal blood vessel penetrating into rhynchocoel; foregut lacunar network present.
Material examined
ZIHU3122, female, 31 July 2003, HK coll., 55 slides, series of 6µm transverse sections of a fragment of body containing cephalic tip; ZIHU3124, female, 1 August 2003, HK coll., 38 + 52 slides, series of 6µm transverse sections of a complete specimen except middle portion; ZIHU3126, female, 1 August 2003, HK coll., 39 slides, 8µm serial transverse sections of a body fragment containing the caudal end.
External features
The body is 3–5 cm long, about 0.7 mm wide, translucent white in colour ( Fig. 15A View FIGURE 15 ). The head is much more transparent than the following portion, wider than the neck; the rhynchodaeum seen through the epidermis is white, cone shaped, tapering anteriorly. When put in a Petri dish with seawater, the animal showed searching behaviour, frequently swinging the head from side to side. The ovaries are tinged with a greyish colour. There is a transparent ‘tail’ region that contains neither intestine nor gonads; it tapers posteriorly to end in a pointed tip ( Fig. 15B View FIGURE 15 ). The tail is at least 10 times as long as the diameter of its most anterior portion.
Body wall, musculature and parenchyma
The ciliated epidermis ( Fig. 16 View FIGURE 16 ) is up to 30–40 µm thick in the brain region, 45–60 µm thick in the foregut region, reduced posteriorly to 10–15 µm thick in the intestinal region. Type 1 cells are confined to the anterior portion of the foregutintestine transitional zone ( Fig. 15C View FIGURE 15 ); type 2 cells predominate throughout the body ( Fig. 15C View FIGURE 15 ); type 3 cells are distributed postcerebrally ( Fig. 15C View FIGURE 15 ); type 4 cells not found; type 5 cells are thinner than type 2 cells, distributed ventrolaterally in the intestinal region ( Fig. 15D View FIGURE 15 ). E (b) = 0.07 (ZIHU3122), 0.11 (ZIHU3124); E (i) = 0.04 (ZIHU3122; the epidermis in the intestinal region was largely sloughed off during fixation or histological preparation in ZIHU3124 and 3126).
The basement membrane is best developed in the brain region, where it reaches a thickness of up to 4 µm thick. At the tip of the head, a thin basement membrane lies between the neuroglandular layer and the bodywall circular muscle layer ( Fig. 17A View FIGURE 17 ). This basement membrane becomes very inconspicuous in front of the proboscis insertion, while another basement membrane appears between the epidermis and the neuroglandular layer ( Fig. 17B View FIGURE 17 ). Postcerebrally, this basement membrane continues between the epidermis and the neurofibrous layer ( Fig. 17C View FIGURE 17 ); it becomes inverted with the latter in the anterior intestinal region, where the body wall is composed, from the surface inwards, of the epidermis, the neurofibrous layer, the basement membrane, and the bodywall musculature ( Fig. 17D View FIGURE 17 ). In the precerebral region, processes extend from inner to outer basement membranes through the neuroglandular layer ( Fig. 17E View FIGURE 17 ); these do not reach the epidermis. A meshlike structure was not found.
The bodywall musculature consists of an outer circular layer and an inner longitudi nal layer, which in the foregut region attain a thickness of 7–15 µm and 12–50 µm, respec tively. Zigzag fibres are absent. The diagonal muscle layer is present but not obvious ( Fig. View FIGURE 17 17C). Dorsoventral muscles were not found. Radial muscle fibres connecting the body wall longitudinal muscle layer and the buccal/foregut wall run through the lumina of the lateral blood lacunae. The foregut and its junction with the intestine are surrounded by a longitudinal muscle layer that is only one or two fibres thick. This splanchnic longitudinal muscle layer is separated from the bodywall longitudinal muscle layer by a thin membrane of connective tissue; the lateral blood lacunae and lateral blood vessels lie outside this splanchnic layer. Posteriorly in the intestinal region, the splanchnic layer disappears except dorsally between the intestine and rhynchocoel, where it remains as a longitudinal muscle plate, terminating anterior to the end of the rhynchocoel.
Parenchymatous connective tissue is inconspicuous throughout the body, except as thin membranes surrounding various organs.
Proboscis apparatus
The rhynchodaeal epithelium is unciliated and shows no significant regional differences in thickness throughout its length; it is generally thinner dorsally and ventrally (20– 30 µm thick) than laterally (35–60 µm thick); acidophilic glandular cells predominate, but in one specimen basophilic glandular cells also occur ( Fig. 17A View FIGURE 17 ). The rhynchodaeum is innervated ventrolaterally on each side by 3–5 nerves from the subepidermal neuroglandular layer; the rhynchodaeal nervous layer is located basal to the glandular epithelium. A definite rhynchodaeal sphincter was not found. A rhynchodaeal caecum is absent; in one specimen, however, a lateral bulge was found on one side, probably caused by contraction during fixation.
The rhynchocoel does not extend to the posterior end of the body. Its wall is composed of separate outer circular and inner longitudinal muscle layers. Posteriorly, it is not developed into a muscular sac. No rhynchocoel caecum was found.
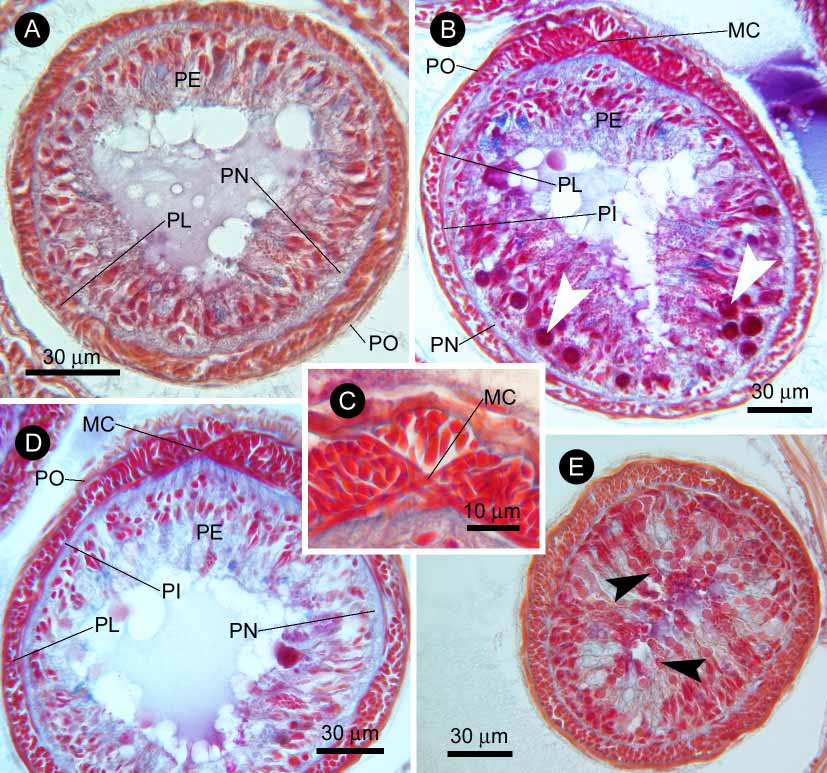
The proboscis insertion is located precerebrally. Four regions can be discerned in the proboscis. The anteriormost region, in retracted position, comprises about 5% of the length of the organ including the proboscis retractor muscle, and is composed of an outer glandular layer, a neural layer, an inner longitudinal muscle layer, an outer circular muscle layer, and an endothelium ( Fig. 18A View FIGURE 18 ). The second portion, about 22% of the length of the organ, has three muscle layers, including an additional outer circular muscle layer between the neural layer and the longitudinal muscle layer ( Fig. 18B View FIGURE 18 ). This region is not radially symmetrical, but has a single muscle cross between the inner and outer circular muscle layers ( Fig. 18C View FIGURE 18 ), although the muscle cross is not always obvious throughout this region. When the muscle cross is viewed in a 12o’clock position, there are additional glandular masses at 4 and 8 o’clock between the outer glandular layer and the neural layer; these glandular masses contain conspicuous acidophilic spherical bodies up to 10 µm in diameter ( Fig. 18B View FIGURE 18 ). The third region, about 27% of the length of the organ, is still composed of three muscle layers with a single muscle cross, but lacks the glandular masses containing spherical bodies ( Fig. 18D View FIGURE 18 ). The last region, about 34% of the length of the organ, has almost the same construction as the first region, but has minute acidophilic spherules near the surface of the epithelium ( Fig. 18E View FIGURE 18 ). It is followed by the proboscis retractor muscle ( Fig. 19A View FIGURE 19 ), 12% of the length of the organ, composed entirely of longitudinal muscle fibres; the proboscis retractor muscle posteriorly attaches to the ventral wall of the rhynchocoel.
Alimentary canal
The mouth opens just behind the cerebral sensory organs. The buccal/foregut wall is densely ciliated, with both acido and basophilic glandular cells; the foregut is about 3 mm long. The intestinal wall contains mainly acidophilic cells, but a small number of basophils cells are also found. The intestine has no lateral diverticula, which, however, appear to be present regionally because the gonads are deeply embedded in the intestinal wall.
Blood system
A pair of lateral cephalic lacunae meets anteriorly above the rhynchodaeal opening. After passing through the cerebral ring, they connect ventrally to form a crosssectionally Ushaped vessel; a thickwalled middorsal vessel is dorsally branched off from the upper surface of the ventral portion of the Ushaped lacuna to enter the rhynchocoel ( Fig. 19B View FIGURE 19 ). The Ushaped lacuna, with its two arms surrounding the cerebral sensory organs ( Fig. 19B View FIGURE 19 ), then divides into two lateral lacunae just anterior to the mouth with the bottom becoming closed between the buccal wall and rhynchocoel. In the foregut region, the thinwalled lacunae lie lateral to the rhynchocoel and above the lateral edges of the foregut, and give rise ventrally to a delicate vascular network that surrounds the foregut laterally, ventrolaterally, and ventrally. In the anterior intestinal region, the vascular network converges posteriorly with a thickwalled lateral blood vessel on each side of the body, while the thinwalled lateral lacunae remain for a short distance, flanked by the rhynchocoel, before ending blindly. The lateral vessels lie ventrolateral to the alimentary canal between the splanchnic and bodywall longitudinal muscle layers. Initially each lateral vessel, together with the bordering splanchnic muscle layer, is distinctly embedded in the gut wall ( Fig. 16 View FIGURE 16 ). Posteriorly, even after the splanchnic muscle layer disappears, the lateral vessels remain in close contact with the intestine.
The middorsal vessel runs inside the rhynchocoel to form a rhynchocoelic villus, then runs down between the rhynchocoel wall and the alimentary canal in the posterior foregut region. Farther backward in the intestinal region, the intestinal wall around the middorsal vessel contains basophilic cells ( Fig. 19A View FIGURE 19 ).
Pseudometameric transverse connections between the lateral and middorsal vessels in the intestinal region were not found.
Nervous system
The brain and lateral nerve cords are situated between the epidermal basement membrane and the bodywall circular muscle layer. A single dorsal commissure, 22–28 µm thick, lies anteriad to the ventral one, 28–30 µm thick. Dorsal and ventral ganglia are almost the same size. Medially each lateral nerve contains a single giant fibre ( Fig. 15D View FIGURE 15 ), about 2 µm in diameter, that can be traced forward to a neural cell body, about 5 µm across, located dorsolaterally in the dorsal commissure. The dorsal ganglion slightly forks posteriorly into upper and lower branches, the latter of which innervates the cerebral sensory organ. The upper middorsal nerve originates in the dorsal commissure and extends posteriorly between the epidermal basement membrane and the bodywall outer circular muscle layer ( Fig. 17C View FIGURE 17 ); farther back, in the anterior portion of the intestine, the middorsal nerve rises to lie between the epidermis and the basement membrane ( Fig. 17D View FIGURE 17 ); this nerve becomes indistinguishable posterior to the rhynchocoel. The middorsal nerve sends numerous branches downwards to the dorsal side of the rhynchocoel wall; thus a lower middorsal nerve seems to be present, but it is not continuous anteroposteriorly.
Frontal organ and cephalic glands A frontal organ and cephalic glands are lacking.
Sense organs On each side of the head there is an epidermal indentation 60 µm long in the antero posterior axis and 80–100 µm long in the dorsoventral axis, lined with very long (about 20 µm) cilia, but no glandular cells. From each indentation, a ciliated canal leads posteroobliquely inward, narrowing from 40–45 µm to about 30 µm in external diameter (10 µm internal diameter), before turning medioventrally to enter a cerebral sensory organ on its dorsolateral surface 1/3 of the way from its anterior end; the canal runs posteriorly inside the organ without branching for the posterior 5/6 of the length of the organ, then terminates in a blind end. A bundle of nerve fibres, innervated from the ventral branch of the posterior end of the dorsal ganglion, runs along the medial side of the canal. Each cerebral sensory organ is an ovoid mass of neuroglandular cells, oval in cross section, about 70–80 µm wide by 90–100 µm high, and about 120–150 µm long; it lies in the cephalic blood lacuna ( Fig. 19B View FIGURE 19 ).
There are neither eyes nor lateral sensory organs.
Excretory system Not found.
Reproductive system
All the three specimens examined were mature females. The ovaries, up to 170 µm in diameter, each containing a single egg, are embedded in the intestinal wall ( Fig. 19A View FIGURE 19 ). A gonoduct leads from each ovary, passing above the lateral nerve cord, then opens dorsolaterally in the epidermis.
Systematic remarks
Hubrechtella (= Coeia ) ijimai ( Takakura, 1922) comb. nov. has not been redescribed since its original description ( Takakura 1922), which provides an account mostly of the features of internal morphology used in the modern diagnosis for the genus Hubrechtella , but not of the characters currently used in distinguishing between species placed in this genus. Unfortunately, the type material of Coeia ijimai Takakura, 1922 is considered to be lost ( Kajihara 2004); thus, it is impossible to identify this species using its internal morphology. Accordingly, I identified my specimens as conspecific with the nominal species Coeia ijimai Takakura, 1922 on the basis only of external characters. However, the resemblance of the shape of the head in living material to Takakura’s (1922) illustration, and the nearness of my sampling site to the type locality, lend support to the identification of my material as Coeia ijimai Takakura, 1922 .
One difference between Takakura’s description and my material is the size of the body; Takakura’s specimens measured over 20 cm in length, while mine do not exceed 5 cm. This difference may be due to the age of the worms. Eventually a neotype must be designated for Coeia ijimai Takakura, 1922 ; however, this will best be done after thorough study of newly collected material from the type locality, or at least closer to it.
The combination of the characters summarised in Table 3 View TABLE 3 enables Hubrechtella ijimai ( Takakura, 1922) comb. nov. to be distinguished from all other species currently placed into Hubrechtella .
Characters and character states:
A: Muscle layers in proboscis: (0) two; (1) three.
B: Bodies in proboscis epithelium: (0) lacking; (1) nematocystlike, rhabditous; (2) spherical.
C: Middorsal blood vessel: (0) does not penetrate the rhynchocoel; (1) penetrates rhynchocoel.
D: Zigzag fibres in bodywall musculature: (0) absent; (1) present.
TABLE 3. Comparison of four characters among Hubrechtella species. Data compiled from Bergendal (1902b), Hylbom (1957), Kirsteuer (1967), Senz (1992, 1993, 2000), Gibson (1979a, b, 1997), Gibson and Sundberg (1999), and Chernyshev (2003).
| Taxa | A | B | C | D |
|---|---|---|---|---|
| H. alba Gibson,1997 | 0 | 0 | 1 | 1 |
| H. atypica Senz,1992 | 0 | 2 | 1 | 0 |
| H. combinata Senz, 1993 | ? | 1 | 1 | 0 |
| H. ehrenbergi Senz,2000 | 0 | 1 | 1 | 0 |
| H. dubia Bergendal,1902 | 0 | 1 | 1 | 1 |
| H. globocystica Senz,1993 | 1 | 2 | 0 | 0 |
| H. indica Kirsteuer,1967 | 0 | 1 | 0 | 0 |
| H. juliae Chernyshev,2003 | 0 | 1 | 1 | 1 |
| H. malabarensis Gibson,1979 | 0 | 1 | 1 | 1 |
| H. queenslandica Gibson,1979 | 0 | 1 | 0 | 0 |
| H. sarodravayensis Kirsteuer,1967 | 0 | 0 | 1 | 0 |
| H. sinimarina Gibson&Sundberg,1999 | 0 | 0 | 0 | 1 |
| H. ijimai (Takakura, 1922) comb. nov. | 1 | 2 | 1 | 0 |
| H. kimuraorum sp. nov. | 1 | 2 | 1 | 0 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Family |
|
|
Genus |
Hubrechtella ijimai ( Takakura, 1922 )
| Kajihara, Hiroshi 2006 |
ijimai ( Takakura, 1922 )
| Kajihara 2006 |
Coeia ijimai
| Takakura 1922 |
Coeia
| Takakura 1922 |
Coeia ijimai
| Takakura 1922 |
Coeia ijimai
| Takakura 1922 |
Coeia ijimai
| Takakura 1922 |
Coeia ijimai
| Takakura 1922 |
Hubrechtella
| Bergendal 1902 |
Hubrechtella
| Bergendal 1902 |