Heth pivari, Phillips & Moulton & Bernard, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4861.4.2 |
|
publication LSID |
lsid:zoobank.org:pub:8B330C85-5B40-48EF-8C17-48332637C1C9 |
|
DOI |
https://doi.org/10.5281/zenodo.4416770 |
|
persistent identifier |
https://treatment.plazi.org/id/8569BD6C-FF96-1260-31A9-1C47FDC2F972 |
|
treatment provided by |
Plazi |
|
scientific name |
Heth pivari |
| status |
sp. nov. |
Heth pivari n. sp.
( Tables 2–4 View TABLE 2 View TABLE 3 View TABLE 4 ; Figs. 2–7 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 )
Type locality and habitat. Florida: Marion County, Silver Springs, Ocala National Forest (29.257726 N, – 81.778702 W), xeric scrub located near Mount Dora Sand Ridge , in intestine of Narceus gordanus GoogleMaps . Other specimens collected: Ridge Manor, Hernando County (28.509794 N, – 82.186692 W); Citrus Springs , Citrus County (29.9786111 N, – 82.4327777 W); and Gainesville , Alachua County (28.707009 N, – 89.395251 W), all in N. gordanus GoogleMaps .
Type designation and deposition. Holotype female (T- 708t) and male paratype (T-6937p), deposited in the USDA Nematode Collection, Beltsville , Maryland . Additional specimens deposited in the nematode collection in the Entomology and Plant Pathology Department, University of Tennessee, Knoxville, Tennessee (University of Tennessee Control Numbers B 1–B24) .
Etymology. We are pleased to dedicate this new species to Dr. Robert J. Pivar, outstanding former graduate student in the Entomology and Plant Pathology Department, University of Tennessee, Knoxville.
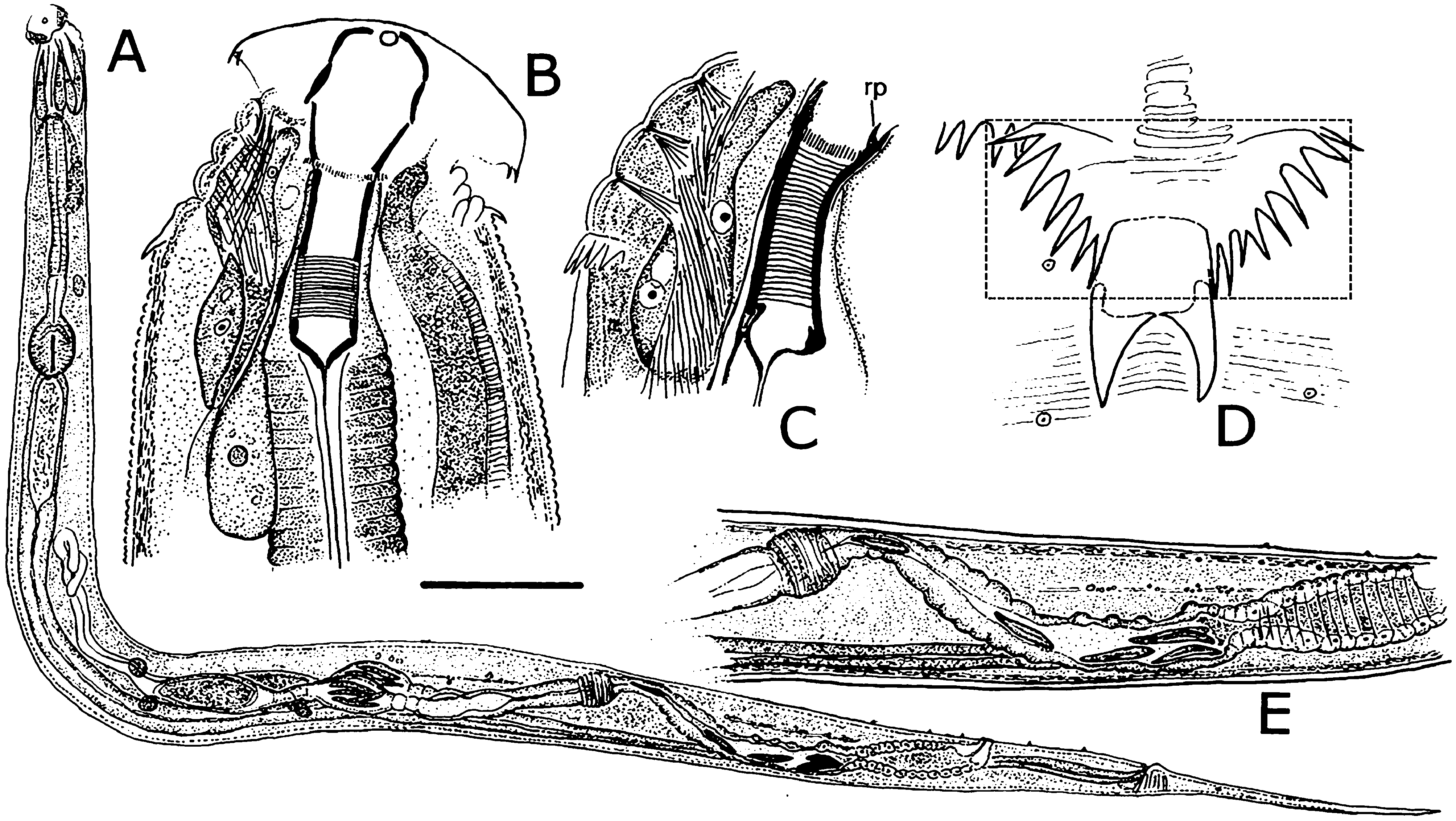
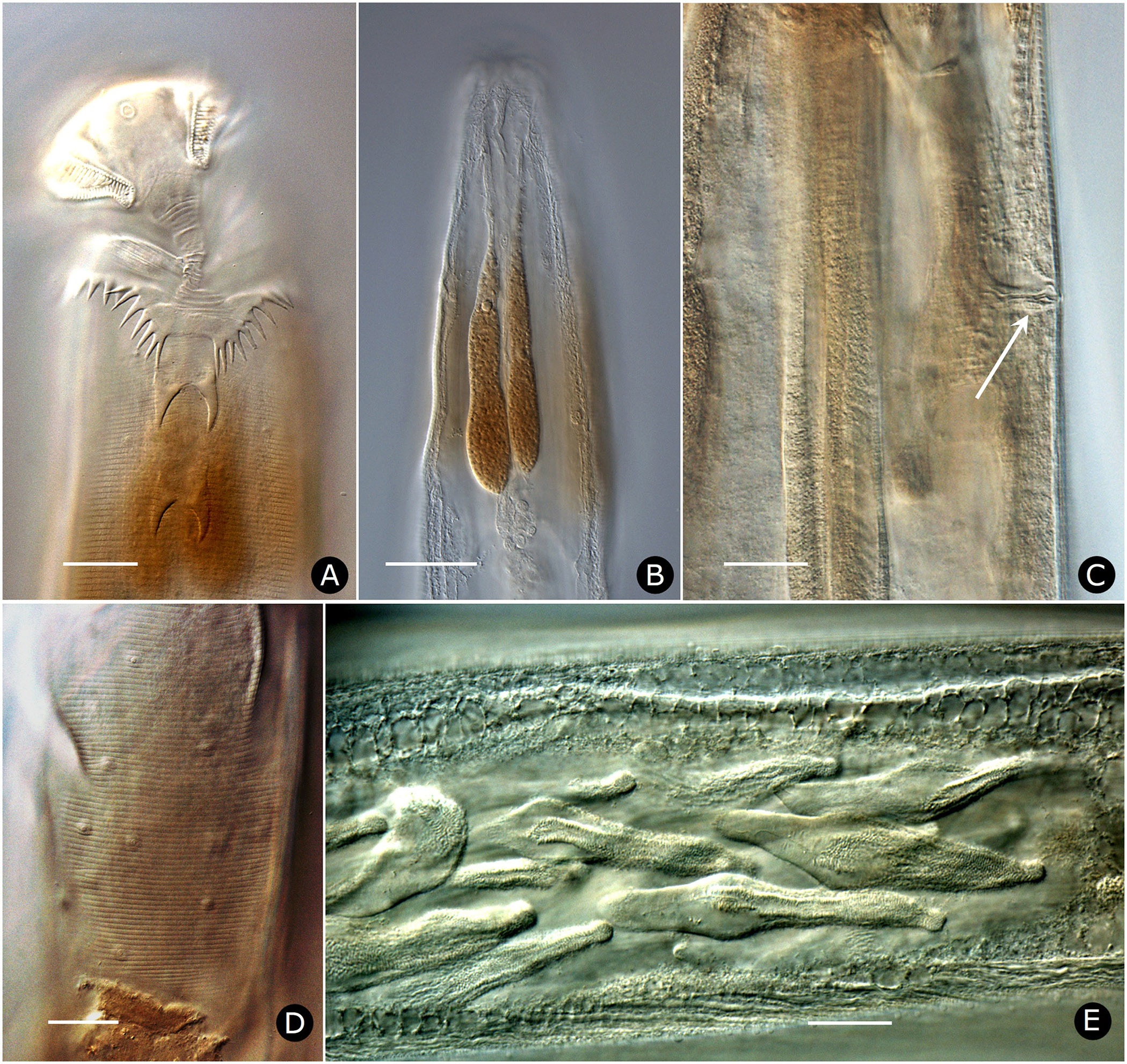
Females. Measurements and ratios given in Table 2 View TABLE 2 . Body cylindrical, white in life, robust, anterior head region broad; maximum diameter near mid-body, then tapering posteriorly and terminating in filiform tail ( Figs. 2A View FIGURE 2 , 7A View FIGURE 7 ). Differentiated lateral field absent. Annules 1–1.5 µm wide posterior to cervical collar, each annule from neck to anal region with numerous minute longitudinal striae ( Fig. 3D View FIGURE 3 ); neck region finely annulated but with weak or no transverse striations ( Figs. 3 View FIGURE 3 A–C). Buccal cavity tubular, expanded anteriorly, much detail obscured by other head structures; anterior and posterior parts divided by circular ring of short, longitudinal striae; posterior part with extremely fine transverse striae about 0.5 µm apart ( Figs. 2B, C View FIGURE 2 ). Amphid apertures circular, 2–4 µm in diameter, located on convex lateral aspect of each pseudolabium ( Figs. 3 View FIGURE 3 A–C). Pseudolabia rectangular in dorso-lateral view ( Fig. 3A View FIGURE 3 ), rounded on outer margin in dorsal view ( Figs. 3 View FIGURE 3 A-C), ornamented with combs and spines; small pectinate combs with 2–3 µm-long bristles on interior lateral, median and cleft margins, and larger combs with 5–7 µm-long bristles continuing along dorsoventral aspect of each pseudolabium; dorsal and ventral bristles rounded distally; length of bristles on each margin approximately equal ( Fig. 3C View FIGURE 3 ). Anterior stomal region often filled with amorphous material ( Fig. 3B View FIGURE 3 ).
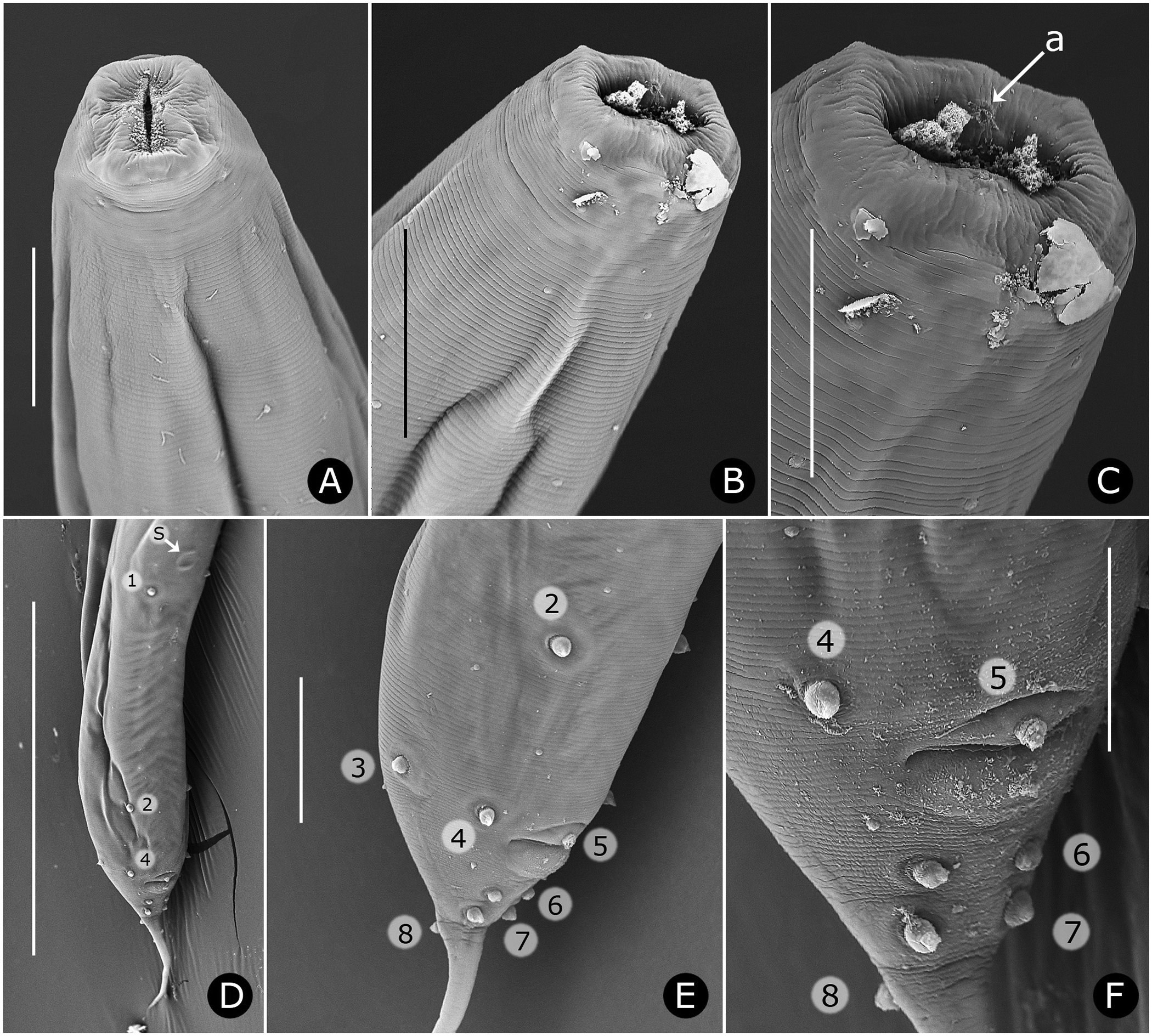
Neck region with four folds anterior to cervical collar, with second, third and fourth folds each containing one smooth, knob-like cervical sensory papilla per quadrant ( Figs. 3B, C View FIGURE 3 ); papillae about 1–2 µm in diameter. Cervical collar with about 72 spines, 5–6 µm long; collar interrupted laterally on each side by shield, edge spines often bi- or trifurcate ( Figs. 3A, D View FIGURE 3 ); shield wider than long ( Figs. 2D View FIGURE 2 , 3A View FIGURE 3 , 6A View FIGURE 6 ). Two lateral pairs of large, acute spines in tandem, 10–13 µm long; anterior pair sometimes connected by a shallow ridge, posterior pair less frequently so ( Figs. 3A, B View FIGURE 3 ). Annulation between each pair of spines irregular and areolated. Knob-like somatic papillae scattered along length of body, denser anterior to vulva ( Figs. 3D View FIGURE 3 , 6D View FIGURE 6 ).
Neck region dorsally and ventrally with retractor muscles attached to body wall at each major fold, integrated with strong longitudinal muscle associated with large nucleus ( Figs. 3B, C View FIGURE 3 ).
Two-part esophagus consisting of procorpus and basal bulb with grinding valve, surrounded anteriorly by six prominent, uninucleate, amber-colored glands ( Fig. 2A View FIGURE 2 ) identical in number and arrangement to those of male ( Figs. 4A, B View FIGURE 4 , 6B View FIGURE 6 ) and one or more small colorless glands ( Fig. 2B View FIGURE 2 ). Secretory-excretory (SE) pore minute, inconspicuous, 167–234 µm from anterior head ( Figs. 2A View FIGURE 2 , 6C View FIGURE 6 ); SE complex fused with nerve ring and anchored dorsally to body wall, identical to that of male ( Fig. 4B View FIGURE 4 ). Four coelomocytes: one in esophageal region, two next to anterior region of intestine or near anterior regions of ovaries, one near spermathecae ( Fig. 2A View FIGURE 2 ). Lateral nerve chord clearly visible, identical to that of male ( Fig. 7H View FIGURE 7 ).
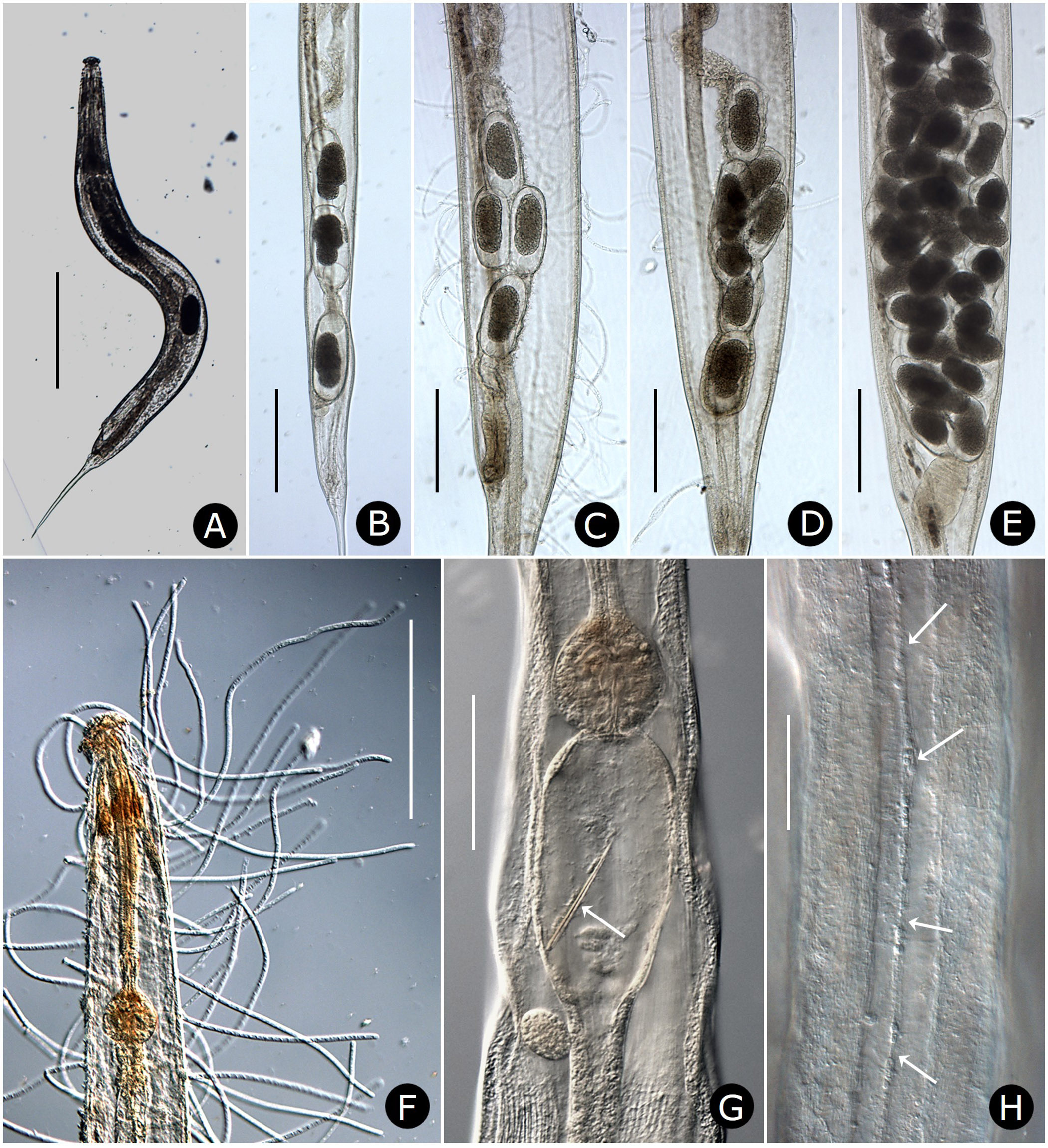
Two gonads, pro-didelphic, reflexed; spermathecae present, filled with large, fusiform or comet-shaped motile sperm ( Figs.2A, E View FIGURE 2 , 6E View FIGURE 6 ). Muscular, spherical sphincter at junction of gonads with oviduct. Uterus prominent, distinctly muscled ( Figs. 2E View FIGURE 2 , 7C, E View FIGURE 7 ). Eggs large, few to moderate in number, eggshells thin, without ornamentation ( Figs. 7 View FIGURE 7 A–E). Vulva near anus. Phasmid apertures minute, pores located just posterior to anus. Tail filiform, attenuated to a fine point.
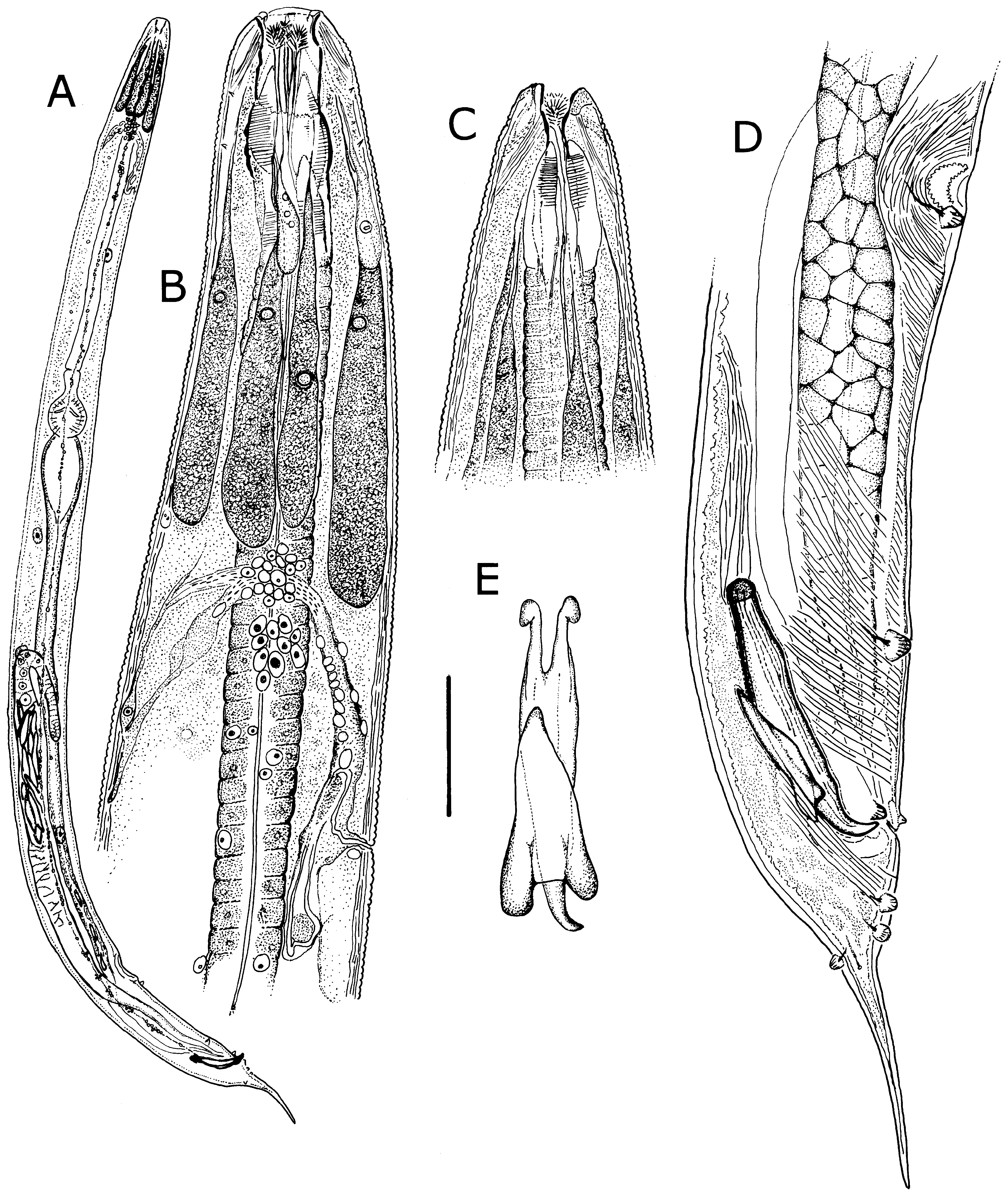
Males. Measurements given in Table 3 View TABLE 3 . Body robust, smaller than females, white, head and neck without ornamentation ( Figs. 4A View FIGURE 4 , 5A, B, C View FIGURE 5 ); lateral field absent; cuticle finely annulated, each annule 1–1.5 µm wide, with scattered, smooth papillae ( Figs. 5B, E View FIGURE 5 ). Stomatal opening longer than wide to slit-like ( Figs. 4B, C View FIGURE 4 , 5 View FIGURE 5 A–C). Stoma tubular, with three feather-like appendages ( Fig. 5B View FIGURE 5 ); stomatal structure partially obscured by tapering, partially striated esophageal collar. Lips finely striated, slightly elevated with four inconspicuous cephalic papillae ( Figs. 5B, C View FIGURE 5 ). Amphid apertures inconspicuous, circular, on inner slope of raised lip region, approximately 3 µm in diameter ( Fig. 5C View FIGURE 5 ). Head region with pairs of subdorsal and subventral muscles attached to stoma and extending obliquely to body wall ( Figs. 4B, C View FIGURE 4 ).
Six prominent amber-brown, uninucleate gland-like somatic extensions of posterior arcade syncytium surrounding anterior part of esophagus and extending to nerve ring ( Figs. 4B View FIGURE 4 , 6B View FIGURE 6 ); at least two much smaller, colorless extensions of the anterior arcade syncytium located near base of stoma ( Fig. 4B View FIGURE 4 ). Esophagus approximately onethird of body length, procorpus muscled; broadly pyriform basal bulb containing grinding valve. Intestine dilated at anterior end, then attenuating to a uniform diameter and in ventral position, crossing testis near ventral sucker and becoming dorsal, then narrowing at cloaca ( Figs. 4A, D View FIGURE 4 ). Secretory-excretory (SE) complex and nerve ring forming a single arching structure ( Fig. 4B View FIGURE 4 ); SE pore inconspicuous, connected to sinuous tube in the SE complex; nerve ring thin, with many associated nuclei, fused with connective tissue extending to dorsal cuticular wall ( Fig. 4B View FIGURE 4 ). Four coelomocytes, one each in esophageal region, in anterior area of intestine, near flexure of testis and near junction of seminal vesicle and vas deferens. Lateral nerve chords clearly visible ( Fig. 7H View FIGURE 7 ).
Reproductive system monorchic, with short flexure in germinal zone. Sperm comet to fusiform-shaped, about 88 µm long × 15 µm at widest point ( Fig. 6E View FIGURE 6 ). Ventral sucker present, 202–330 µm anterior to cloacal opening, flanked by first pair of large pre-cloacal papillae ( Figs. 4D View FIGURE 4 , 5D View FIGURE 5 ). Region between sucker and cloaca with scattered somatic papillae of 1–2 µm diameter. Spicules equal, fused for distal three-fourths; each spicule with distinct capitulum, apical fused portion arcuate ventrad, tapering to a point; gubernaculum with pointed proximal end, distal end broad and bilobed ( Figs. 4D, E View FIGURE 4 ). Cloacal lips smooth. Seven pairs of genital papillae, each with several conspicuous internal striae or minute tubes; one median ventral papilla. Caudal papilla pattern as follows: first pair slightly posterior to ventral sucker; second pair about 30 µm anterior to cloaca, distant from third pair; pairs 3 and 4 slightly anterior to cloaca, pair 3 ventral, pair 4 lateral; single medial papilla (5) on anterior cloacal lip; posterior to cloaca, two ventral pairs (6, 7) and one subdorsal pair (8) ( Figs. 4D View FIGURE 4 , 5 View FIGURE 5 D–F).
Differential diagnosis. Females of Heth pivari n. sp. are characterized by smooth, knob-like cervical and somatic papillae, shallow cuticular shields, continuous cuticular collars with approximately 72 subequal spines, and two pairs of paired anterior and posterior lateral spines. Males lack ornate cuticular ornamentation, have a narrowed stomal opening and smooth somatic papillae, but otherwise are similar to other described males.
The morphology of females of H. pivari n. sp. is most similar to those Heth spp. having a continuous collar of cervical spines with a spiny lateral shield. Heth pivari n. sp. is distinct from H. mauriesi Adamson, 1982 , its closest geographic neighbor, in female length (2,190 –4,483 µm vs. 1,575 –2,000 µm in H. mauriesi ), smooth cervical and somatic papillae (multi-cusped in H. mauriesi ), and a trapezoidal shield interrupting the cervical collar (shield replaced by two stout spines in line with the smaller collar spines in H. mauriesi ) ( Figs. 1A, B View FIGURE 1 ). Among the other subtropical and tropical North American species, H. tuxtlensis ( Fig. 12B View FIGURE 12 ) is most similar to H. pivari n. sp. in having a continuous cervical collar, the presence of lappets that usually do not overlap the anterior lateral spines, absence of lateral alae, and a finely striated body. Heth pivari n. sp. and H. tuxtlensis are easily differentiated by the number of cervical spines (ca. 72 for H. pivari n. sp., ca. 100 for H. tuxtlensis ), a shallow cervical shield in H. pivari n. sp., (more pronounced in H. tuxtlensis ), more bifurcated lappet spines in H. tuxtlensis as compared to fewer cervical spine bifurcations in H. pivari n. sp. and smaller anterior and posterior lateral spines (10–13 µm long) compared to the larger and more robust spines of H. tuxtlensis (anterior spine length 58–67 µm, posterior spine length 82–94 µm posterior spines).
Females of H. pivari n. sp. resemble those of H. taybaci ( Vietnam) in that the shields have similar shapes. The posteriormost spines of the cuticular collar nearly reach the base of the anterior lateral spines, and both have smooth somatic papillae. Heth pivari n. sp. has fewer collar spines (approximately 72) compared to about 88 around the circumference of H. taybaci . Heth pivari n. sp. also differs in that the anterior and posterior lateral spine pairs are smaller than those of H. taybaci . Males of H. pivari n. sp. have smaller spicule arcs (mean 109 µm) than H. taybaci (136 µm) but have longer bodies ( Heth pivari n. sp. males 1,897 –2,609 µm, H. taybaci 1,520 µm).
Two additional Heth species with a continuous shield-like collar occur in the western hemisphere: H. insularis ( Brazil) and H. orthopori ( Paraguay) . In both of those species the lateral spine pairs are broadly joined at their bases. All other Heth species in this group are located in Australia, New Zealand, and Asia. Heth pivari n. sp. has short lappets whose posteriormost spines do not reach the anterior lateral spines, whereas in H. costata , H. impalutiensis , H. sutherlandi , H. taynguyeni , and H. vietnamensis the spines of the much longer lappets overlap the anterior lateral spines. Heth baudini and H. dimorphum have shields in which length and width are about equal, whereas in H. pivari n. sp. the shallow shield width is about twice its length. The type species, H. juli , has a “W” shape as opposed to a trapezoidal shape in H. pivari n. sp. Finally, H. xaniophora has prominent lateral alae and robust anterior and posterior lateral spines, whereas H. pivari n. sp. lacks lateral alae and has small anterior and posterior lateral spines.
Molecular analysis. With the addition of H. pivari n. sp. and H. mauriesi , nine Heth species were included in a 28S rDNA tree ( Fig. 8 View FIGURE 8 ). Heth pivari n. sp. and two Mexican species, H. gordae and H. xarochae , grouped together with high support. The other six, including H. mauriesi , had no support except for H. initiaensis - H. konoplevi . The molecular relationship of H. pivari n. sp. to H. gordae and H. xarochae is supported by geographical location but not by morphological similarity.
| USDA |
United States Department of Agriculture |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Rhigonematomorpha |
|
SuperFamily |
Ransomnematoidea |
|
Family |
|
|
Genus |