Gephyrocharax machadoi, Ferreira & Faria & Ribeiro & Santana & Quagio-Grassioto & Menezes, 2018
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4415.1.8 |
|
publication LSID |
lsid:zoobank.org:pub:6FF3114F-87A5-4E93-897F-46AD7CE023E4 |
|
DOI |
https://doi.org/10.5281/zenodo.5974855 |
|
persistent identifier |
https://treatment.plazi.org/id/03AFFF4E-3034-FFAD-EE98-9D1818E5F818 |
|
treatment provided by |
Plazi |
|
scientific name |
Gephyrocharax machadoi |
| status |
sp. nov. |
Gephyrocharax machadoi , new species
Fig. 1 View FIGURE1
Gephyrocharax View in CoL sp. Lima & Ribeiro, 2011: 152 (occurrence in the rio Paraguai basin; based on MZUSP 90000).
Holotype. CPUFMT 2429 (37.6 mm SL, male), Brazil, Mato Grosso, Tangará da Serra, Rio Sepotuba (14°30’05”S, 57°34’39”W), W.P. Troy, 15 October 2010. GoogleMaps
Paratypes. All from Brazil, Mato Grosso: CPUFMT 2428, 6, 33.9–39.3 mm SL; MZUSP 123130, 1 View Materials , 36.8 View Materials mm SL, collected with holotype GoogleMaps ; CPUFMT 1463, 2, 40.9–41.4 mm SL, 1 c&s, 42.2 mm SL, Nova Marilândia, Rio Sepotuba (14°25’29”S 57°11’06”W), G.M.M. Figueiredo, 19 March 2011 GoogleMaps ; CPUFMT 1464, 3, 25.7–26.5 mm SL, Tangará da Serra, Rio Ararão (14°30’23”S 57°34’00”W), G.M.M. Figueiredo, 21 March 2011 GoogleMaps ; CPUFMT 2387, 5, 29.1–36.6 mm SL, Tangará da Serra, Rio Sepotuba (14°30’06”S 57°34’39”W), W. Troy, November 2011 GoogleMaps ; MZUSP 90000, 2, 17.5–31.5 mm SL, Tangará da Serra, Rio Sepotuba (14°30'04"S, 57°34'38"W), H.A. Britski, O. Froehlich & F. Marques, 10 March 2002 GoogleMaps .
Diagnosis. Gephyrocharax machadoi differs from all congeners, except Gephyrocharax major , by presenting two modified scales on the ventral caudal-fin lobe: a larger, sexually dimorphic scale, slightly superior and anterior in position, followed by a smaller, posterior and ventrally placed accessory scale (vs. a single modified pouch scale without ventrally placed accessory scales). Gephyrocharax machadoi differs from G. major by presenting a gap (more conspicuous in mature males than in mature females) (see sexual dimorphism below) between the second and third ventral procurrent caudal-fin rays (vs. second and third ventral procurrent caudal-fin rays near each other or fused distally); premaxilla with tricuspid teeth (vs. tetra- to pentacuspid teeth); and body depth 21.5–25.4% of the SL (vs. 25.9–36.8% in SL).
Description. Morphometric and meristic data of holotype and paratypes presented in Table 1. Body laterally compressed, moderately elongate, largest specimen 41.4 mm SL. Greatest body depth situated at dorsal-fin origin. Dorsal profile of head slightly convex from margin of upper lip to tip of supraoccipital spine; straight from tip of supraoccipital spine to dorsal-fin origin; straight along dorsal-fin base; straight from posterior terminus of dorsalfin base to adipose-fin insertion, and slightly concave from latter point to caudal-fin origin. Ventral profile of body convex from tip of lower jaw to anal-fin insertion, slightly concave along anal-fin base and slightly concave ventral to caudal peduncle.
Dorsal-fin rays ii, 8* (17). Length of first unbranched dorsal-fin ray less than one-half length of second unbranched ray. Dorsal-fin origin located posterior to vertical crossing the origin of the anal-fin origin. Dorsal-fin first pterygiophore inserted behind sixteenth neural spine of caudal vertebra. Adipose fin present. Pectoral-fin rays i, 11* (17). Pelvic fin rays i,5,i* (17), longest ray not reaching anal-fin origin. Anal fin with three unbranched rays followed by 30* (8), 31 (7) or 32 (2) branched rays. Caudal-fin forked; lobes similar in size. Principal caudal-fin rays i,17,i* (17).
Scales cycloid. Lateral line complete, with 46 (2), 47* (4), 48 (5), 49 (3), 51 (1) or 52 (2) perforated scales. Six (17)* longitudinal rows of scales between dorsal-fin origin and lateral line, five (17)* between lateral line and pelvic-fin origin. Predorsal scales in an irregular series of 26 (1), 27 (3), 28 (3), 29* (6), 30 (2) or 31 (2).
Circumpeduncular scales (17). Single row of five to seven scales extending along anal-fin base. Basal portion of both caudal-fin lobes covered by medium-sized scales, about same size as those present on caudal peduncle (see Sexual Dimorphism section for more details).
Mouth superior, lower jaw projecting slightly anterior to tip of upper jaw. Premaxilla with two rows of teeth ( Fig. 2 View FIGURE 2 ). Outer tooth row aligned in gentle arch, with 3 (7) or 4* (10) tricuspid teeth. Inner premaxillary tooth row with 5 (8) or 6* (9) tricuspid teeth; both outer and inner tooth rows with median cusps largest. Maxilla with 1 (1), 2 (12) or 3* (3) large tricuspid teeth with median cusps slightly more developed. Dentary with five large anterior teeth with three cusps, with median cusps largest, followed by smaller conic teeth.
First gill arch with 12 (5) gill rakers on hypobranchial and ceratobranchial, 6 (5) rakers on epibranchial, and 1 (5) on cartilage between ceratobranchial and epibranchial.
Color in alcohol. Males and females with approximately the same color pattern. Overall ground coloration yellowish tan. Head and body of specimens retaining guanine on scales, therefore somewhat silvery. Dorsal surface of head and lips with dense concentration of dark chromatophores. Scattered dark chromatophores covering 1/3 of opercular region, upper and lower lips, nostrils and on maxilla, lateral ethmoid, frontal, parietal and occipital process. Dark chromatophores concentrated on predorsal scales. Concentration of chromatophores decreasing progressively from middorsal region to lateral line, where limited to edges of scales. Chromatophores in scales of abdominal region below lateral line. Few chromatophores present between lateral line and anal fin. Humeral blotch vertically elongate on fourth and fifth perforated lateral line scale, extending two or three scale rows above and one scale row below lateral line. Midlateral stripe on body extending from humeral blotch to caudal peduncle, located above lateral line. Large dark blotch on caudal peduncle. Dorsal, pectoral, pelvic, and caudal fins hyaline, with scattered dark chromatophores outlining rays and forming straight lines. Dark chromatophores concentrated along distal borders of interradial membranes of anal-fin base. Adipose fin pale, with small, dark chromatophores concentrated on posterior base of fin.
Distribution. Gephyrocharax machadoi is known only from tributaries of the rio Sepotuba, upper rio Paraguai Basin, Mato Grosso, Brazil ( Fig. 3 View FIGURE 3 ).
Etymology. Gephyrocharax machadoi is named in honor of the Brazilian ichthyologist Francisco de Arruda Machado, Universidade Federal de Mato Grosso, for his great contribution in the conservation of neotropical freshwater fishes, especially in the Mato Grosso state.
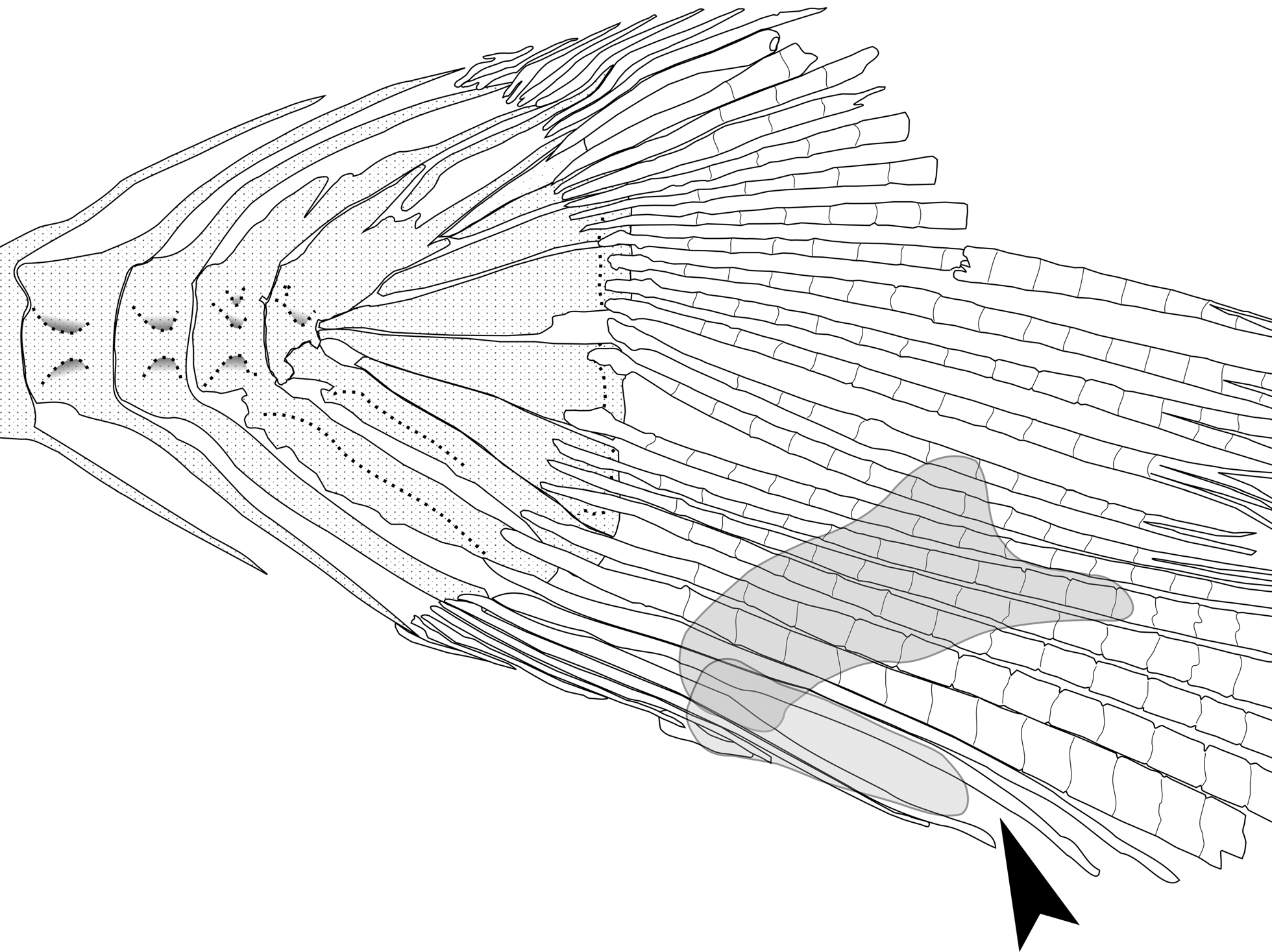
Sexual dimorphism. Sexually mature males with two modified scales on the caudal-fin ventral lobe. First modified scales larger, more anterior and superior in position (slightly below lateral line), triangular in shape and covering almost the entire portion of the caudal-fin ventral lobe ( Fig. 4A View FIGURE 4 ). Females also present a modified scale, with size and position similar to the modified scale abovementioned, however, this scale present a rounded margin ( Fig. 4B View FIGURE 4 ). The second modified caudal-fin scale in males is smaller, more posterior and inferior in position, and cover the posterior half of procurrent caudal-fin rays 1, 2 and 3.
Both sexually mature males and females present a gap between ventral procurrent caudal-fin rays 2 and 3. This gap is more developed in sexually matures males than in females ( Fig. 5 View FIGURE 5 ).
Sexually mature males have bony hooks on rays of pelvic, anal, and caudal fins. All branched pelvic-fin rays have small, slender, anterolaterally oriented hooks along nearly entire length of each ray, usually one hook per segment. Anal-fin bony hooks very small and located on the largest unbranched ray up to the sixth branched ray; usually, there are nine hooks per ray. Caudal fin with three or four small, slender, anterodorsally oriented hooks, on the branched portions of the fourth to sixth rays.
Histological analysis. Gephyrocharax machadoi has spermatozoa with ovoid nuclei, and packs of spermatozoa were observed inside the female’s ovary ( Fig. 6 View FIGURE 6 ).
Spermiogenesis description. In early spermatids the nucleus is spherical and displays granular chromatin homogenously distributed ( Fig. 7A–B View FIGURE 7 ). The centriolar complex and the flagellum lie laterally to the nucleus and it is anchored to the plasma membrane ( Fig. 7A–C View FIGURE 7 ). The proximal centriole is anterior and oblique in relation to distal centriole ( Fig. 7C View FIGURE 7 ). The distal centriole differentiates into basal body that organizes the axoneme of the forming flagellum ( Fig. 7C–D View FIGURE 7 ). The centriolar complex migrates towards the nucleus. The nucleus moves up to the centriolar complex ( Fig. 7B–C View FIGURE 7 ), where a depression, the nuclear fossa, is formed in nuclear outline at the centriolar complex point ( Fig. 7C View FIGURE 7 ). The organelles are dispersed around the nucleus at opposite area to the centriolar complex ( Fig. 7D–E View FIGURE 7 ). The chromatin gradually starts a synchronous condensation process ( Fig. 7A–E View FIGURE 7 ). The nucleus rotates towards the forming flagellum and assumes an eccentric position ( Fig. 7D–E View FIGURE 7 ). Also, the cytoplasm shifts towards the flagellum, giving rise to the midpiece ( Fig. 7A–E View FIGURE 7 ). The distal centriole is anchored to the plasma membrane, and the cytoplasm encircles the initial portion of the flagellum forming the cytoplasmic canal ( Fig. 7B View FIGURE 7 ). The midpiece is irregular and contains the mitochondria and vesicles ( Fig. 7A, E View FIGURE 7 ). The axoneme of the flagellum is formed by the classical microtubule disposition 9+2 (not shown).
Spermatozoon description. The nucleus displays granular chromatin ( Fig. 8A–I View FIGURE 8 ), is elongated in direction of the flagellum and drop-shaped in cross section ( Fig. 8A–B View FIGURE 8 ). The nucleus is split into an anterior larger and a posterior smaller, which involves the initial portion of the flagellum ( Fig. 8C–E View FIGURE 8 ). The nuclear outline has a double concavity located in a superolateral position in relation to the nucleus, the nuclear fossa ( Fig. 8F View FIGURE 8 ). The proximal centriole is partially inside the nuclear fossa and the distal centriole is totally outside it ( Fig. 8F View FIGURE 8 ). Within the centriolar complex, the proximal centriole has an oblique position forming an acute angle in relation to the distal centriole ( Fig. 8F View FIGURE 8 ). The midpiece is irregular and contains mitochondria and vesicles ( Fig. 8A–B View FIGURE 8 ). The mitochondria are elongated and irregular-shaped, without branches, and distributed through the midpiece and the nuclear periphery ( Fig. 8A–B, G–I View FIGURE 8 ). Two mitochondria are found in the spermatozoon (I–J). A cytoplasmic projection without organelles, called citoplasmatic sleeve, is found at the end of the midpiece ( Fig. 8L View FIGURE 8 ). Only one flagellum is present ( Fig. 8A–B, D View FIGURE 8 ) with the classical pattern of the axoneme (9 + 2, 9 pairs of peripheral microtubules and 2 central microtubules) ( Fig. 8I View FIGURE 8 ).
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Gephyrocharax machadoi
| Ferreira, Katiane M., Faria, Érika De, Ribeiro, Alexandre C., Santana, Júlio C. O., Quagio-Grassioto, Irani & Menezes, Naércio A. 2018 |
Gephyrocharax
| Lima & Ribeiro, 2011 : 152 |