Epibolus pulchripes (Gerstäcker, 1873), Gerstacker, 1873
|
publication ID |
https://doi.org/ 10.5281/zenodo.276689 |
|
DOI |
https://doi.org/10.5281/zenodo.5612676 |
|
persistent identifier |
https://treatment.plazi.org/id/03BC87EA-FF8C-3143-FF22-FF6702F4FC53 |
|
treatment provided by |
Plazi |
|
scientific name |
Epibolus pulchripes (Gerstäcker, 1873) |
| status |
|
Epibolus pulchripes (Gerstäcker, 1873)
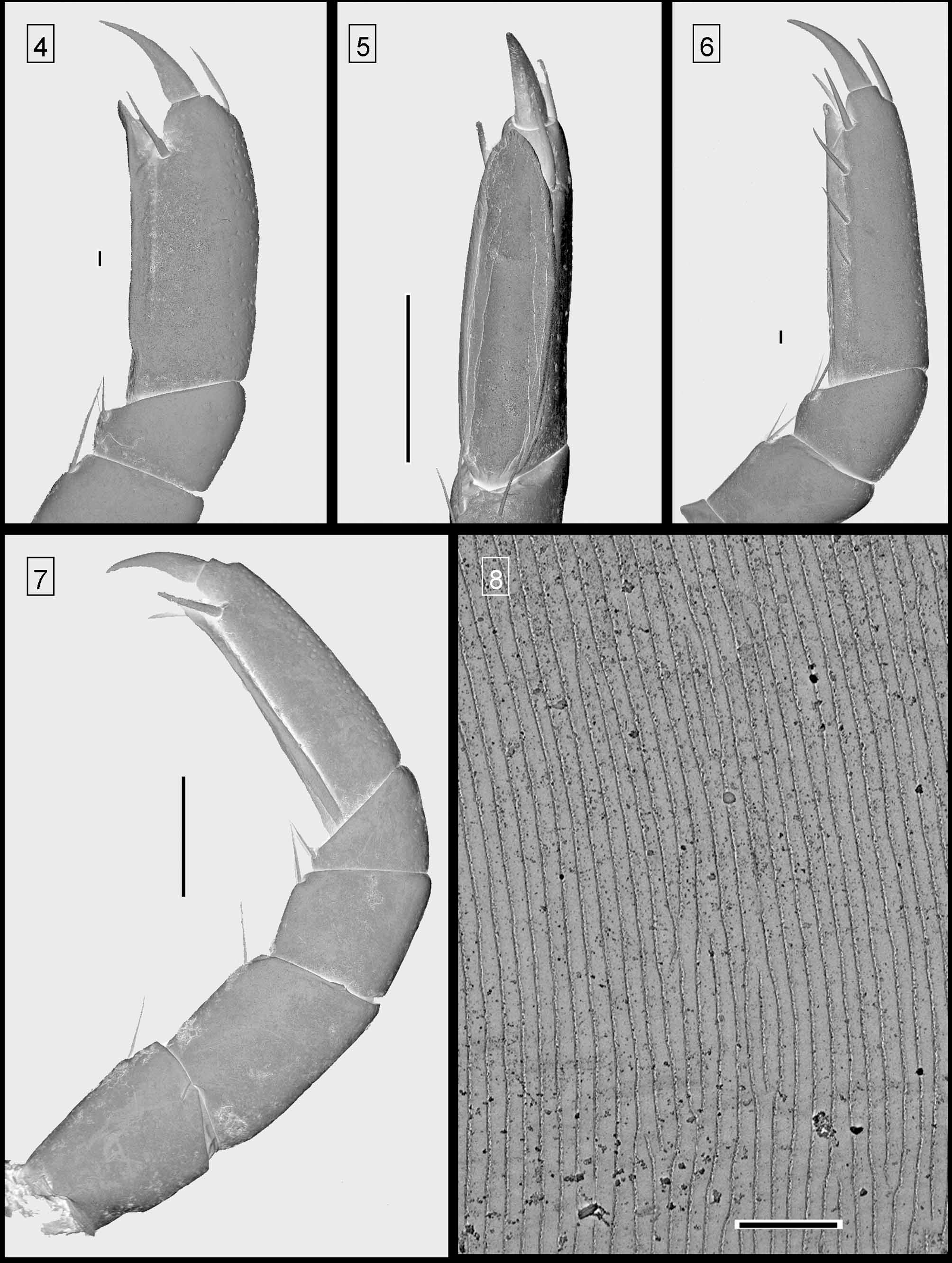
( Figs 6 View FIGURES 4 – 8 , 9–10 View FIGURES 9 – 10 , 12 View FIGURES 11 – 12 , 78–82 View FIGURES 78 – 80 View FIGURES 81 – 82 )
Spirobolus pulchripes Gerstäcker, 1873
Spirobolus proporus Attems, 1896 , synonymized by Enghoff (1977) Trigoniulus ruspolii Silvestri, 1896 , synonymized by Enghoff (1977) Epibolus pulchripes: Cook (1897)
Trigoniulus bravensis Silvestri, 1897 , new synonym
Metiche bravensis: Attems (1909) View in CoL
Metioche attemsi Kraus, 1958 , synonymized by Enghoff (1977) Metiche tanganyicense Kraus, 1958 View in CoL , synonymized by Enghoff (1977) Callipodolus pulchripes: Hoffman & Keeton (1960)
Metiche mossambicense Lawrence, 1967 View in CoL , new synonym Epibolus bravensis: Enghoff (1977)
Epibolus mossambicensis: Enghoff (1977)
Metiche tanganyciense [sic!]: Banerjee (1980)
Epibolus pulchripes bravensis: Cecchi & Chelazzi (1984) . Material examined (numerous specimens from Kenya and Tanzania not listed): 5 3 Somalia, (Gelib) Alessandra, July 1937, F. Bigi leg. (MSNF).
Diagnosis. See that of genus.
Descriptive notes. Usually 52 podous rings (27 out of 43 specimens studied by Enghoff 1977), exceptionally 50, 51 or 53 podous rings, no apodous rings. Dhaenens & VandenSpiegel (2006) observed only 52 podous rings in the population they studied.
Male sexual characters. Male coxae 6–7 unmodified. Posterior gonopods unmistakable because of the moveable lateral appendix ( Figs 78 View FIGURES 78 – 80 , 81–82 View FIGURES 81 – 82 ). See, e.g., Kraus (1958), Hoffman (1962), Enghoff (1977) and Dhaenens & VandenSpiegel (2006) for descriptions of E. pulchripes gonopods.
Female sexual characters. Distal margin of lateral coxosternal extensions projecting as smoothly rounded, subhemicircular lobe ( Fig. 12 View FIGURES 11 – 12 ). Vulvae ( Figs 79–80 View FIGURES 78 – 80 ) complicated; operculum particularly poorly sclerotized; oral valve divided into two sclerites and in addition with a hood-shaped appendix; aboral valve constricted ca. at same level as subdivision of oral valve. Crest strongly protruding. ( Enghoff (1977) described and illustrated the vulva of E. pulchripes but mistook the apical appendix for the operculum and translated the French term ‘cimier’ as ‘ridge’, whereas I use ‘crest’ here.)
Notes to the new synonyms. Enghoff (1977) provisionally upheld E. bravensis and E. mossambicensis as distinct species, but there is little justification for this.
E. bravensis was distinguished from E. pulchripes by 1) the number of ocelli per eye: ca. 35 in bravensis (Silvestri 1897) , vs., 38–57 in male E. pulchripes , 2) the shape of the anterior gonopod sternum which in bravensis has a deep, acute incision of the basal margin (Silvestri 1987: fig. 6). The studied specimens from Alessandra, Somalia are perfectly normal E. pulchripes in all characters, including the number of ocelli. They do have a pronounced basal incision in the anterior gonopod sternum but so do other specimens from Tanzania which I have studied after 1977. The purported differences between pulchripes and bravensis can therefore be dismissed as either intraspecific variation (the gonopod character) or a possible inaccuracy of observation by Silvestri (number of ocelli). Alessandra is now known as Labadaad and lies at 0°30’ N, 42°45’ E, quite close to (160 km) the type locality of E. bravensis, Brava , now known as Bawaawe and lying at 1°6’N, 44°2’ E. Cecchi & Chelazzi (1984) took a step in the direction of synonymizing bravensis under pulchripes by treating it as a subspecies (without any argumentation for doing so).
E. mossambicensis was kept separate exclusively on the basis of its very slender posterior gonopod fingerformed process. Although no additional specimens from Mozambique have come to hand, and although no specimen with an equally slender process has been found anywhere, the variability in shape of the finger-shaped process observed in Tanzanian specimen ( Figs 78 View FIGURES 78 – 80 , 81–82 View FIGURES 81 – 82 , Dhaenens & VandenSpiegel 2006: fig. 3) is so great that separate status of mossambicensis cannot be maintained.
Distribution. E. pulchripes including its new synonyms bravensis and mossambicensis , is widely distributed in eastern Africa: from Bawaawe, Somalia, in the north to Island of Mocambique in the south although the vast majority of finds are from Kenya and Tanzania. Most, but not all occurrences are close to the Indian Ocean coast. See map in Enghoff (1977).
Further notes. Epibolus pulchripes is by far the best studied among the species treated here and is subject of several non-taxonomic papers. Thus Wood et al. (1975) studied the defensive secretion of E. pulchripes (reporting that it can be sprayed by the animal as far as 40 cm), Banerjee (1980) studied population characteristics of the species, Cecchi & Chelazzi (1984) described its internal genital organs and spermatogenesis, and Dhaenens & VandenSpiegel (2006) provided information on post-embryonic development and reproduction.
According to United Nations Environment Programme (1998: 42), E. pulchripes is common on the East African coast and “very seldom damages living plants, but instead is a very valuable agent in humus formation”. In this publication, E. pulchripes is in this respect contrasted with the giant spirostreptid Archispirostreptus gigas ( Peters, 1855) with which it often coexists: “Like many other giant millipedes, A. gigas can cause serious localized seasonal damage to crops and small forestry seedlings. In dry conditions, millipedes turn to living plants as a source of food and they burrow down and seek shelter in crevices. On the other hand, under wet conditions their populations appear to increase explosively but they seem to confine their diet to leaf litter and other dead plant matter.”
In an account of a reforestation project at Bamburi Nature Trail near Mombasa, Kenya, E. pulchripes was recorded to play an important role, as shown by the following quote from http://www.cosy.sbg.ac.at/~zzspri/travels/BANweb/bantrail.html (accessed 25 September 2009): “ Casuarina and Conocarpus are evergreen trees that constantly drop and renew "needles" and leaves. Thus the dropping foliage was covering the rocky ground at a steady pace. However, the break down of foliage's by micro-organism was prolonged because of the high content of tannin in Casuarinas "needles''. The process of releasing the confined nutrients to other organisms was slowed down and the establishment of subsequent plant species was delayed thus. A lucky incident solved this problem when the red legged Mombasa trains ( Epibolus pulchripes ) happily started feeding on dry Casuarina "needles''. The compost bacteria in millipedes' dropping converted needles into the needed humus. These useful animals were collected from coastal bushes and there was an instant success as the millipedes multiplied fast in the young forest. The leaf litter was slowly reduced and a layer of humus thickened underneath. The millipedes had found a land of milk and honey having the forest floor for them only but this happy state of affairs did not last long. Soon they became part of the food chain too as white tailed mongooses and civet cats started eating millipedes as they came in the forest on their own.” See also http://blog.earthshope.org/wp-content/uploads/2009/02/jp-morgan-2008-jlessay.pdf and http://www.iaphworldports.org/HoustonPresentations/IAPH2007-Kahumbu.pdf (both accessed 25 September 2009).
E. pulchripes is a popular pet millipede (e.g., Decker & Pfeifle 2008) and has received a variety of common names, e.g. Mombasa train, Tanzanian red-legged millipede, and giant red-legged millipede in English, Afrikanischer Lackschnurfüßer and Rotbeiniger Schnurfüsser or Rotfuss-Schnurfüsser in German. In Kenya it is eaten by the elephant shrew Rhynchocyon chrysopygus View in CoL , but is avoided by the dwarf mongoose Helogale parvula View in CoL although this mongoose will readily eat Archispirostreptus gigas View in CoL (unpublished obervations reported by Wood et al. (1975)).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Tribe |
Pachybolini |
|
Genus |
Epibolus pulchripes (Gerstäcker, 1873)
| Enghoff, Henrik 2011 |
Epibolus pulchripes bravensis:
| Cecchi & Chelazzi 1984 |
Epibolus bravensis:
| Enghoff 1977 |
Epibolus mossambicensis:
| Enghoff 1977 |
Metiche mossambicense
| Lawrence 1967 |
Callipodolus pulchripes:
| Hoffman & Keeton 1960 |
Metioche attemsi
| Kraus 1958 |
Metiche tanganyicense
| Kraus 1958 |
Metiche bravensis:
| Attems 1909 |
Epibolus pulchripes:
| Cook 1897 |
Trigoniulus bravensis
| Silvestri 1897 |
Spirobolus proporus
| Attems 1896 |
Trigoniulus ruspolii
| Silvestri 1896 |