Ecdyonurus dispar gratificus, Martynov, Alexander V. & Godunko, Roman J., 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3666.4.5 |
|
publication LSID |
lsid:zoobank.org:pub:034BC0E4-ACC4-4451-914B-804D9726FB2A |
|
DOI |
https://doi.org/10.5281/zenodo.6162161 |
|
persistent identifier |
https://treatment.plazi.org/id/039B87FB-FF93-FFC2-FF49-CAA7FE1EF8D4 |
|
treatment provided by |
Plazi |
|
scientific name |
Ecdyonurus dispar gratificus |
| status |
subsp. nov. |
Ecdyonurus dispar gratificus ssp. nov.
Figures 1–55 View FIGURES 1 – 8 View FIGURES 9 – 13 View FIGURES 14 – 16 View FIGURES 17 – 20 View FIGURES 21 – 30 View FIGURES 31 – 41 View FIGURES 42 – 51 View FIGURES 52 – 55
Ecdyonurus sp. nov.: Martynov 2012: 132; Martynov 2013: 15–16, fig. 5
Description. Male imago (main part of adults were reared from larvae) ( Figs. 1, 4, 6 View FIGURES 1 – 8 , 9–13 View FIGURES 9 – 13 ). Size: body length 9.7– 11.0 mm, forewing length 10.5–11.4 mm, cerci length 20.0– 24.5 mm (approximately 1.9–2.2 times longer than body).
General color of body pale, yellowish brown to light brown ( Fig. 1 View FIGURES 1 – 8 ). Head light brown to brown, with paler (dirty yellow to light brown) clypeal part. No stripes or bands on the head. Eyes divided by a narrow gap, narrower than central ocellus; unicolorous grayish-blue to grayish-black, sometimes with one whitish strip laterally. Ocelli black at the base, whitish apically. Antennae light brown, paler basally ( Figs. 1, 6 View FIGURES 1 – 8 ).
Prothorax dirty brown, paler centrally ( Fig. 1 View FIGURES 1 – 8 ). Mesothorax light brown to brown dorsally; diffuse brownish spot around anterolateral scutal suture. Prelateroscutum and posterior arc of prealar bridge with elongated dark brown spots. Scutellum dark brown. Metathorax brown to dark brown. Laterally thorax pale; mesothorax yellowish-brown, with brown to black spots on katepimeron and katepisternum; ventrally intensively brown.
Forelegs generally darker than middle and hind ones ( Fig. 1 View FIGURES 1 – 8 ). Forefemora brown proximally, distinctly darker distally (approximately 1/2 to 2/3 of femora length dark brown). Foretibia and tarsi darker than distal part of femur or slightly paler. Middle and hind legs yellow to yellowish-brown; tibia darker proximally; tarsal segment V darker than other ones. All tarsal claws brown.
Wings hyaline, transparent, with yellowish-brown to brown venation; no dark maculation around transversal veins; costal and subcostal field distally yellowish-milk colored in pterostigmatic area; pterostigma with several, mainly simple, cross veins (only 3–4 transversal veins are forked). Wings with typical Heptageniidae venation.
Abdominal terga yellow to yellowish-brown; tergum I unicolorous brown, distinctly darker than others; terga II–VIII with broad diffuse brownish spot centrally, pair of small elongated pale spots situated centrally (passing occasionally on pale tine strokes, at least on terga II–VII); tergum IX unicolorous brown; tergum X unicolorous yellowish-brown. Terga II–VIII laterally yellowish-brown, with distinct brown oblique stripes connected dorsally in posterior margin of segment ( Fig. 4 View FIGURES 1 – 8 ). Sterna yellowish to dirty-yellowish, with two unclear brownish spots. Reddish to violet nerve ganglia visible on the sterna II–VIII. Cerci dark brown to yellow, distinctly paler apically. Joints of segments blackish.
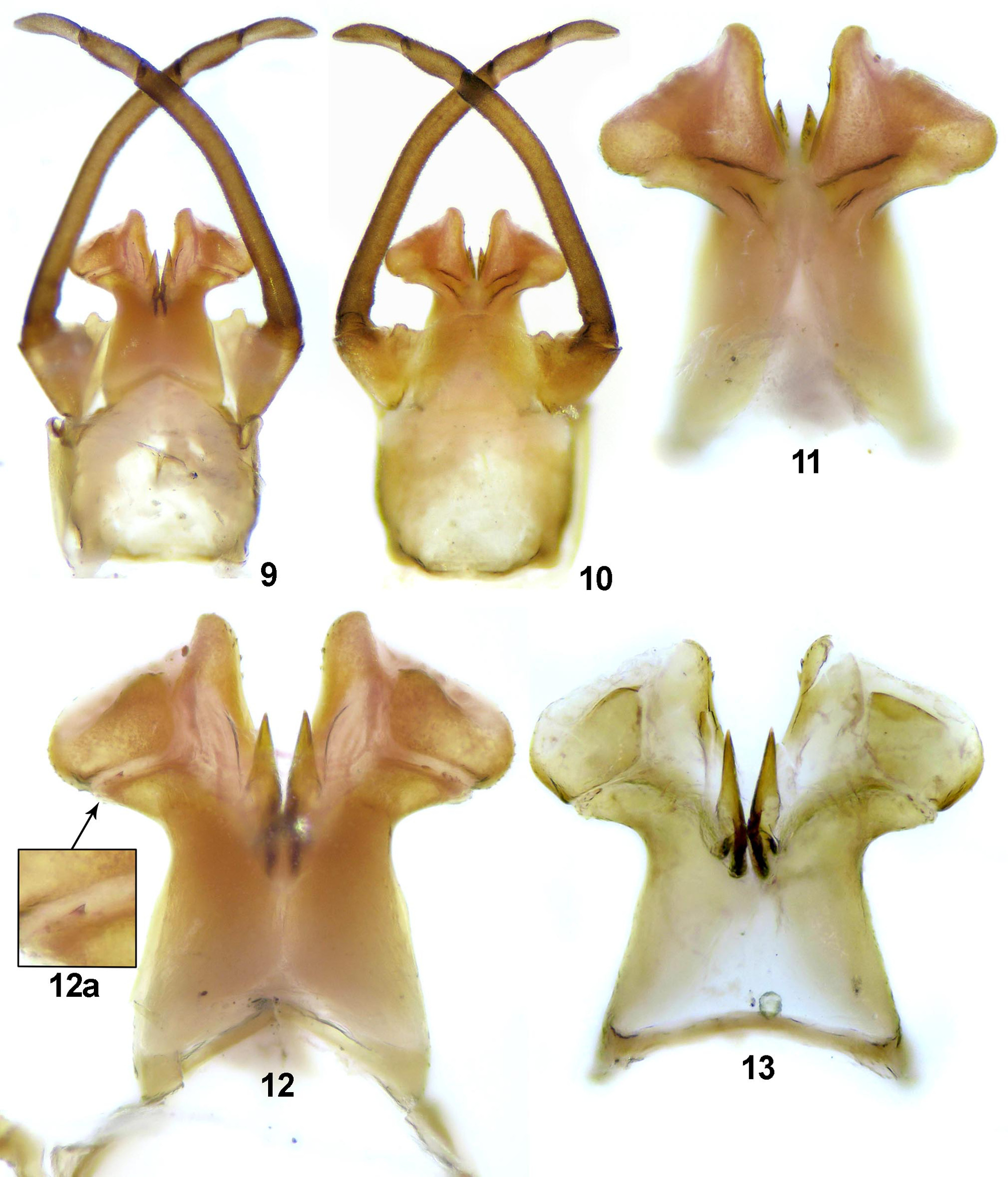
Genitalia. Styliger yellowish with distinctly brown forceps, with two small, not curved, symmetrical and rounded protuberances near forceps segment I; segment II with small hump at inner margin basally ( Figs. 9–10 View FIGURES 9 – 13 ). Penis lobes yellowish-brown, moderately expanded, not stretched laterally, with rounded outline distally, broadly trapezoidal. Basal sclerite massive, well separated from lateral sclerite with 1–3 small teeth ( Figs. 12 View FIGURES 9 – 13 a). Lateral sclerite large, tapered proximally; the widest part of lateral sclerite situated at its distal end; the tip of lateral sclerite distinctly broad. Apical sclerite remarkably wide, straight; the tip distinctly projecting above the lobes; inner margin covered with small spines ( Figs. 11–13 View FIGURES 9 – 13 ). Titilators brown, wide.
Female imago ( Figs. 2–3, 5, 7–8 View FIGURES 1 – 8 ). Size: body length 9.7–12.4 mm, forewing length 10.6–13.5 mm, cerci length 14.8–21.8 mm (approximately 1.4–1.7 times longer than body).
Head yellowish-brown with reddish maculation; eyes and basal part of ocelli unicolorous grayish-black ( Fig. 7 View FIGURES 1 – 8 ). Antennae yellow to brown, paler basally.
Thorax dorsally and ventrally brown to dark brown, with spots situated similar to those in male imago. Lateral portions of thorax yellow to brown ( Figs. 2, 7 View FIGURES 1 – 8 ). Legs slightly more robust than in male imago. Forelegs brown to dark brown, distinctly darker than middle and hind ones. Middle and hind legs yellowish-brown; femora darker than tibia and tarsi. Wings hyaline, transparent, with well visible brown venation. Costal and subcostal field distally milky colored, with numerous cross veins in pterostigmatic area.
Color pattern of abdomen similar to those in male imago ( Figs. 3, 5 View FIGURES 1 – 8 ), occasionally more distinct. Blackish neural ganglia visible on sterna I–VII. Abdominal terga yellowish-brown, with unclear reddish maculation. Subgenital and subanal plates as in Fig. 8 View FIGURES 1 – 8 ; subgenital plate widely rounded, occasionally reaching articulation of segment IX; subanal plate distinctly bent, with smoothly rounded outline distally. Cerci dark brown basally (at least of 1/3 of the length), yellowish-brown to yellow distally.
Male subimago ( Figs. 14–15 View FIGURES 14 – 16 ). Size: body length 9.9–10.4 mm, forewing length 10.5–11.0 mm, cerci length 12.1–13.6 mm (approximately 1.2–1.3 times longer than body).
Head yellow to yellowish-brown, with grayish maculation. Eyes grayish-black apically; two distinct blackish stripes laterally, one of them at its base ( Fig. 14 View FIGURES 14 – 16 ). Ocelli as in male imago. Antennae grayish-brown, paler basally.
Prothorax yellowish-gray to brown, darker laterally. Mesothorax dorsally pale, yellow to yellowish-brown (especially in central part). Spots arrangement the same than in male imago. Lateral portions of mesothorax yellowish, with darker suturae. Metathorax yellowish-brown ( Fig. 14 View FIGURES 14 – 16 ). Ventral side of thorax yellowish-brown to brown.
Forelegs darker than middle and hind ones. Forefemora yellowish-gray to yellow proximally; intensively brown distally. Middle and hind legs uniformly yellowish-gray to yellow, tarsi occasionally darker.
Wings dull yellowish-gray to brownish, relatively transparent. Venation yellowish-gray to brown; in some specimens, transversal veins darker than longitudinal, brown to black.
Color pattern of abdominal terga similar to those in male imago, occasionally slightly paler. Lateral part of terga I–VIII with distinct brown stripes connected distally in posterior part of segments ( Fig. 15 View FIGURES 14 – 16 ). Sterna slightly darker than terga. Styliger plate yellowish-gray to yellow, forceps and protuberances of styliger plate slightly or distinctly darker, yellowish-brown to dark brown. The typical shape of penis already well apparent. Cerci brown to dark brown, distinctly paler apically.
Female subimago ( Fig. 16 View FIGURES 14 – 16 ). Size: body length 9.8–12.6 mm, forewing length 10.5–14.5 mm, cerci length 10.1– 12.1 mm (approximately 1.1–1.2 times longer than body).
Head and antennae yellow to yellowish-brown.
Dorsal side of prothorax yellow, whitish posteromedially. Mesothorax pale, whitish-yellow to yellow, with brownish spots around suturae. Metathorax yellowish-brown.
Legs of the same arrangement and color pattern as in male subimago. Wings uniformly dull yellowish-gray to brownish, relatively transparent, with brown to dark brown venation. Abdominal color pattern the same as in male subimago. Cerci dark brown ( Fig. 16 View FIGURES 14 – 16 ).
Mature larva ( Figs. 17–51 View FIGURES 17 – 20 View FIGURES 21 – 30 View FIGURES 31 – 41 View FIGURES 42 – 51 ). Size: body length (slightly differs between males and females): in male 8.8–11.8 mm; cerci length 8.0– 9.5 mm, paracercus length 8.0– 9.1 mm; in female 10.0– 14.4 mm, cerci length 9.0– 12.3 mm, paracercus length 8.0– 10.5 mm.
General body color yellowish-brown to brown ( Figs. 17–20 View FIGURES 17 – 20 ). Abdominal segments of mature larvae with well visible dark pattern on lateral sides of terga, similar to adults ( Fig. 21 View FIGURES 21 – 30 ).
Head yellowish-brown to brown, with apparent light (yellow) pattern consisting of: two central spots near fore margin; one central and two elongated transversally spots near antennal bases; lateral margins with two further spots near eyes; one central and two lateral spots near posterior margin. Head rectangular with the widest part at the level of the eyes ( Fig. 19 View FIGURES 17 – 20 ). Antennae yellow to light brown.
Mouthparts: Labrum not wide, only slightly stretched laterally; one row of 6–9 median bristles dorsally typical of the subgenus Ecdyonurus (cf. Belfiore & Buffagni 1994) ( Figs. 31–32 View FIGURES 31 – 41 ). Hypopharynx without specific features, generally with long hair on the outer margin and distal part, typical of the subgenus Ecdyonurus . Mandibles (n = 20) with 9–11 prosthecal bristles. Maxillae (n = 20) (characters listed by Haybach 1999): number of comb-shaped bristles (N_CBS) = 13–18 (mainly 15−18) (+ 1–3 pointed bristles); number of teeth on 5th comb-shaped bristle (N_TCB5) = 9–12 (mainly 9–10); number of hairs on dorsal upper side (N_DOR) = 1–11 (mainly 3–5); outer margin of maxillae without hairs (N_OUT) = 0; number of hairs on ventral basal part of maxillae (N_VEN) = 5–17 (mainly 5–8); number of hairs at the base of maxillary palps (N_PLBas) = 9–13; number of hairs at outer base of the first segment of maxillary palps (N_PLH) = 11–16; number of setae on the outer margin of the first segment of maxillary palps (N_PLS) = 33–40; number of setae on the inner side of the first segment of maxillary palps (N_PLP) = 35–75 (mainly 40–60). Labial palps with numerous hairs on the dorsal side of its first segment (N_LPH) = 15–24 (mainly 17–20) arranged in 1–2 (rarely 3) rows. Glossae slightly stretched laterally ( Fig. 33 View FIGURES 31 – 41 ).
Pronotum yellowish-brown to brown; several pale spots situated centrally and laterally; lateral projection paler ( Fig. 19 View FIGURES 17 – 20 ). Pronotum extended laterally and rounded. Lateral projection relatively short, asymmetrical, sometimes slightly curved outwards at the apex ( Figs. 42–45 View FIGURES 42 – 51 ). Apex of lateral projection bluntly pointed or pointed; the width/ length ratio of semipronotum to caudal projection (cf. Bauernfeind & Humpesch 2001) is 3.2–3.8. Meso- and metathorax yellowish-brown to darker brown; longitudinal pale (yellowish) lines in middle and lateral sides ( Fig. 19 View FIGURES 17 – 20 ).
Legs yellowish-brown to brown; yellow pattern dorsally similar to "broken" cross (four lateral spots on a dark field) ( Fig. 34 View FIGURES 31 – 41 ). The nymphs' forelegs slightly darker than other ones. Tibiae yellow, with distinct brownish smudge centrally; tarsi yellowish-brown, with light smudge centrally (two brownish rings basally and apically). The length/ width ratio of metafemora is 2.8–3.2. Bristles on dorsal surface of femora of various types ( Figs. 35–40 View FIGURES 31 – 41 ): pointed or bluntly pointed apically bristles, with strongly convergent margins (dominant type); bluntly pointed apically bristles, with slowly convergent margins or spatula-like elongated bristles, with sub-parallel margins. Outer margin of femora with row of long slender bristles and regular row of small pointed spines ( Fig. 41 View FIGURES 31 – 41 ) (in contrast to E. aurantiacus (Burmeister, 1839) , cf. Haybach 1999: 128, fig. 4f; Haybach & Thomas 2002: 87, fig. 34). Tarsal claw brown, with 2–3 teeth (rarely up to 4–5 teeth) ( Figs. 46−49 View FIGURES 42 – 51 ).
Abdominal terga yellowish-brown to brown with contrast yellow pattern. Tergum I with broad light spot centrally; terga II and III with two spots centrally, and two spots laterally near posterior margin of segment. The variations of color pattern of the terga IV–X are shown in Figs. 17, 18, 20 View FIGURES 17 – 20 . The nymphs with distinct hypodermal markings on lateral sides of abdominal terga as in imago (dark oblique stripes) ( Fig. 21 View FIGURES 21 – 30 ). Posterior margin of terga with large pointed teeth alternating with small ones ( Figs. 50–51 View FIGURES 42 – 51 ). Numerous small submarginal spines arranged in 2–3 rows. Surface of terga with numerous fain hairs.
Sterna yellowish-brown: sternum I unicolorous with unclear light spots centrally; sterna II–IV with brown elongated spots laterally near anterior margin of segment; sterna V and VI with color pattern resembling that of the previous segments, but including broader spots laterally, divided towards the posterior margin of segment; sterna VII and VIII with diffuse brown spots laterally along the sides of segment, and one small spot (mainly on sternum VIII) frontally; (in male) sternum IX with dark brown spot frontally, and two spots laterally along the sides of segment (these spots surround the segment); forceps anlagen yellow colored centrally, with broad brown spots near bases of each forceps; (in female) sternum IX unicolorous brown, slightly paler distally. Well visible violet nerve ganglia on the segments II–VII ( Figs. 22–23 View FIGURES 21 – 30 ).
Gills yellowish to light brown with distinct dark tracheation, sometimes with a conspicuous pigmented zone extending over the central and proximal part. Gill I with a specific shape: distinctly broad and asymmetrical; relatively long with divergent proximally margins in basal part (at least throughout of 1/2–2/3 of gill plate length); apical part broadly rounded; the widest part situated in 2/3 to 3/4 of gill length ( Figs. 24–25 View FIGURES 21 – 30 ). Gill IV distinctly wide and asymmetrical, occasionally outer margin conspicuously convex ( Figs. 26–28 View FIGURES 21 – 30 ). Gill VII elongated and asymmetrical, without a tuft of tracheal filaments ( Figs. 29–30 View FIGURES 21 – 30 ). Larger specimens have more rounded outline of gills ( Figs. 25, 28, 30 View FIGURES 21 – 30 ).
Cerci and paracercus yellowish-brown to brown, paler distally.
Egg. Measurements: length 160–175 μm; width 135–150 μm. Egg slightly elongated ( Fig. 52 View FIGURES 52 – 55 ). Chorionic surface covered by numerous attachment structures (represented by knob-terminated coiled threads – KCTs) and small tubercles, typical of the Ecdyonurus eggs (cf. e.g. Gaino & Rebora 2003; Kłonowska-Olejnik et al. 2007); significant concentrations of relatively large KCTs attachment structures (diameter 4.0–4.4 μm, distance between them 1.8–7.0 μm) on the whole surface of the egg. Many small rounded tubercles (0.7–1.1 μm) spaced at a distance of 0.05–0.7 μm, and scattered on the chorionic surface between KCTs attachment structures ( Figs. 53–54 View FIGURES 52 – 55 ). The same concentration of KCTs attachment structures on both eggs poles. Three to six micropyles visible in subequatorial area. Sperm guide ovoidal or slightly elongated, 8.0–10.0 μm in length and 5.2–7.0 μm in width. Micropylar rim absent; margins of micropyles covered by a few sparsely distributed tubercles ( Fig. 55 View FIGURES 52 – 55 ).
Etymology. The new subspecies is named in honor of Dr. Vladimir V. Martynov, father and first scientific adviser of the first author (name originates from the Latin adjective gratificus, i.e. appreciative).
Material examined. Types. HOLOTYPE: male imago, UKRAINE, Donetsk Ridge, Donetsk Region, Artemivs’kyi District, vicinity of Debaltsevo urban settlement, (border of Donetsk and Lugansk Regions), Bulavyna River in the forest, 48º18’55’’N 38º26’07’’E, 22.08.2011, Martynov A.V. leg.
PATARYPES: UKRAINE, 4 larvae (were mounted on slides with Liquide de Faure), Donetsk Ridge, Donetsk Region, Artemivs’kyi District, vicinity of Debaltsevo urban settlement, (border of Donetsk and Lugansk Regions), Bulavyna River in the forest, 48º18’55’’N 38º26’07’’E, 13.07.2008, Martynov A.V. leg.; 1 larva, ibid, 3.05.2010, Martynov A.V. leg.; 4 larvae, ibid, 16.05.2010, Martynov A.V. leg.; 6 larvae (one mounted on slide with Canada balsam), ibid, 30.05.2010, Martynov A.V. leg.; 24 larvae, ibid, 11.06.2010, Martynov A.V. leg.; 41 larvae, ibid, 1.07.2010, Martynov A.V. leg.; 1 female subimago, ibid, 17.07.2010, Martynov A.V. leg.; 14 larvae, ibid, 18.07.2010, Martynov A.V. leg.; 19 larvae, ibid, 1.08.2010, Martynov A.V. leg.; 43 larvae (11 were mounted on slides with Canada balsam), 2 female subimagoes, ibid, 20.08.2010, Martynov A.V. leg.; 7 larvae, 1 female subimago, ibid, 19.09.2010, Martynov A.V. leg.; 2 larvae, ibid, 4.06.2011, Sergeev M.E. leg.; 6 larvae, ibid, 18.06.2011, Martynov A.V. leg.; 35 larvae, 3 female imagoes, ibid, 8.07.2011, Martynov A.V. leg.; 36 larvae, 9 female subimagoes, 9 male subimagoes, 20 female imagoes, 10 male imagoes, ibid, 22– 23.08.2011, Martynov A.V. leg.; 1 larva, ibid, 15− 16.10.2011, Martynov A.V. leg.; 2 larvaе, Donetsk Ridge, Donetsk Region, Amvrosiiv’kyi District, vicinity of Blahodatne village (2 km N −W from the village), stream in the forest – left tributary of Krynka river, 47º53’41’’N 38º27’00’’E, 20.11.2011, Martynov A.V. leg.; 6 larvaе, Donetsk Ridge, Lugansk Region, Antratsytivs’kyi District, vicinity of Fashchevka village, stream in the forest, 48º15’34’’N 38º35’53’’E, 13.07.2008, Martynov A.V. leg.; 2 larvae (were mounted on slides with Canada balsam), ibid, 1.08.2010, Martynov A.V. leg.; 1 larva (mounted on slide with Liquide de Faure), Lugansk Region, Antratsytivs’kyi District, vicinity of Bokovo-Platove village, Kripen’ka River in the forest, 48º08’01’’N 39º01’29’’E, 24.10.2008, Martynov A.V. leg.; 1 larva (mounted on slide with Liquide de Faure) Donetsk Ridge, Luhansk Region, Antratcitivs’kyi District, vicinity of Faschevka village, head of the Mius River in the forest, 48º16’00’’N 38º34’29’’E, 13.07.2008, Martynov A.V. leg.
Further material examined (no types). 2 larvae, Donetsk Ridge, Donetsk Region, Artemivs’kyi District, vicinity of Debaltsevo urban settlement, (border of Donetsk and Lugansk Regions), Bulavyna River in the forest, 48º18’55’’N 38º26’07’’E, 17.04.2010, Martynov A.V. leg.; 7 larvae, ibid, 2.05.2010, Martynov A.V. leg.; 38 larvae, ibid, 30.05.2010, Martynov A.V. leg.; 35 larvae, ibid, 11.06.2010, Martynov A.V. leg.; 34 larvae, ibid, 1.07.2010, Martynov A.V. leg.; 53 larvae, ibid, 18.07.2010, Martynov A.V. leg.; 62 larvae, ibid, 1.08.2010, Martynov A.V. leg.; 22 larvae, ibid, 20.08.2010, Martynov A.V. leg.; 1 larva, ibid, 13.03.2011, Martynov A.V. leg.; 8 larvae, ibid, 29.04.2011, Martynov A.V. leg.; 10 larvae, ibid, 4.06.2011, Sergeev M.E. leg.; 1 larva, ibid, 18.06.2011, Martynov A.V. leg.; 183 larvae, ibid, 8.07.2011, Martynov A.V. leg.; 12 larvae, ibid, 15– 16.10.2011, Martynov A.V. leg.; 1 larva, Donetsk Ridge, Donetsk Region, Artemivs’kyi District, vicinity of Debaltsevo urban settlement, (border of Donetsk and Lugansk Regions), stream in the forest – right tributary of Bulavyna River in the forest, 48º18’46’’N 38º25’58’’E, 8.07.2011, Martynov A.V. leg.; 1 larva, Donetsk Ridge, Donetsk Region, Amvrosiiv’kyi District, vicinity of Blahodatne village (2 km N −W from the village), stream in the forest – left tributary of Krynka river, 47º53’41’’N 38º27’00’’E, 20.11.2011, Martynov A.V. leg.; 9 larvaе, Donetsk Ridge, Donetsk Region, Amvrosiiv’kyi District, vicinity of Blahodatne village, Krynka river, 47º53’37’’N 38º30’17’’E, 18.05.2011, Sergeev M.E. leg.; 9 larvaе, Donetsk Ridge, Donetsk Region, Shakhtars’kyi District, vicinity of Oleksandrivs’ke urban settlement, stream in the forest, between settlement and Uglegirs’ka Zapadna mine, 48º17’05’’N 38º16’29’’E, 25.09.2011, Martynov A.V. leg.; 1 larva, Donetsk Ridge, Donetsk Region, Shakhtars’kyi District, stream between Oleksandrivs’ke and Bulavyns’ke urban settlements, 48º15’52’’N 38º18’30’’E, 25.09.2011, Martynov A.V. leg.; 8 larvaе, Donetsk Ridge, Donetsk Region, Shakhtars’kyi District, vicinity of Illinka village, stream in the forest within Skeleva gully, 48º15’44’’N 38º26’42’’E, 7−8.08.2011, Martynov V.V. leg.; 1 larva Donetsk Ridge, Donetsk Region, Shakhtars’kyi District, vicinity of Ol‘khovatka urban settlement, stream in the forest, 2 km W from Kamianka village, 48º15’15’’N 38º27’44’’E, 25.09.2011, Martynov A.V. leg.; 29 larvae, Donetsk Ridge, Luhansk Region, Antratcitivs’kyi District, vicinity of Faschevka village, head of the Mius River in the forest, 48º16’00’’N 38º34’29’’E, 13.07.2008, Martynov A.V. leg.; 4 larvaе, Donetsk Ridge, Lugansk Region, Antratsytivs’kyi District, vicinity of Fashchevka village, stream in the forest, 48º15’34’’N 38º35’53’’E, 1.08.2010, Martynov A.V. leg.; 1 larva, Donetsk Ridge, Lugansk Region, Antratsytivs’kyi District, vicinity of Ivanivka village, stream in the forest, 48°15’38’’N 38°58’36’’E, 29.04.2012, Martynov A.V. leg.
Affinities. Taxonomical relationships of the new subspecies with E. dispar dispar . We defined the taxonomic status of the new taxon, described here, as the subspecies of widespread European species E. dispar .
Both adult and larval stages of the new subspecies is rather similar to corresponding stages of nominal E. dispar . The color pattern of male and female adults is similar in both subspecies, and cannot be used for the separation of these taxa (the same were noted for all Mediterranean species of the subgenus Ecdyonurus by Haybach & Thomas 2002: 90). The nominal subspecies shows large range variations of thorax and abdominal segments coloration (especially of abdominal terga II–VIII). Some of these variations of color pattern are overlapped with those described above for E. dispar gratificus ssp. nov. Both subspecies are characterized by a moderately expanded, not stretched laterally penis lobes with rounded distally outline; inner margin of the segment II of forceps with small, but well visible hump basally (this feature was earlier noted by Jacob, 1972: 85 for the “ dispar -Komplex”).
A further similarity of both subspecies is observed in the larval characteristics, such as: (i) some overlapping of number of groups of setae, bristles and hairs on maxillae and labial palpi (especially for N_CBS, N_TCB5, N_DOR, N_VEN and N_LPH); (ii) coloration of abdominal terga and sterna (cf. Figs. 17, 18, 20–23 View FIGURES 17 – 20 View FIGURES 21 – 30 ; fig. 2c, d by Haybach 1999: 122 showing sterna of Central European E. dispar ); (iii) coloration of tarsi (cf. Fig. 34 View FIGURES 31 – 41 ; for nominal subspecies e.g. Bauernfeind 1997: 419; Haybach 1999: 132; Bauernfeind & Soldán 2012).
Finally, in contrast to other representatives of the subgenus Ecdyonurus , both subspecies of E. dispar can be characterized by significant concentrations of relatively large KCTs attachment structures on the whole surface of the egg. For the nominal subspecies of E. dispar such chorion structure was described for the first time by Degrange (1960: 45) under the name E. fluminum , and later confirmed and depicted on the material from Germany and Western Turkey (Haybach 2003: 50, fig. 9; our Figs. 52–55 View FIGURES 52 – 55 ).
At the same time the larvae of E. dispar gratificus ssp. nov. can be easily distinguished from nominal subspecies by: (i) the number of some groups of setae, bristles and hairs on maxillae, i.e. N_PLBas = 9–13 (in contrast to up to 26 in E. dispar dispar ); N_PLS = 33–48 (in contrast to up to 25–26 in E. dispar dispar ) (Haybach 1999: 132, 134); (ii) the shape of pronotal lateral projection, i.e. sometimes slightly curved outwards at the apex, bluntly pointed or pointed apically (in contrast to broadly rounded apically in nominal E. dispar , cf. Thomas 1968: 58; Bauernfeind 1997: 423; Bauernfeind & Humpesch 2001: 71, fig. 204); (iii) the shape of scales of dorsal surface of femora (clearly stouter and acutely pointed in E. dispar dispar , cf. Bauernfeind 1997: 421); (iv) the shape of gills (especially gill I) (compare our Figs. 24–30 View FIGURES 21 – 30 and fig. 6e by Haybach 1999: 133 for E. dispar dispar ).
The male genitalia of the new subspecies markedly differ from E. dispar dispar by the general shape of more slender and broadly trapezoidal penis lobes, with apical sclerite distinctly projecting above the lobes; presence of symmetrical, not curved protuberances near forceps segment I (cf. our Figs. 9–10 View FIGURES 9 – 13 and fig. 6 by Kimmins 1942: 498; fig. 2 by Thomas 1968: 57; fig. 485 by Bauernfeind & Humpesch 2001: 138–140). Clear differences between both subspecies can be also documented in the shape and color of the male imago eyes, i.e. in contrast to new subspecies, the eyes of E. dispar dispar touch in the middle and are unicolorous gray.
Taxonomical relationships of the new subspecies with closely related species of Ecdyonurus s. str. The larvae of both subspecies of E. dispar , as also the larvae of E. belfiorei Haybach & Thomas, 2002 and E. solus , occupy a relatively isolated position, and can be separated from remaining representatives of the subgenus Ecdyonurus by the presence of distinctly broad, more or less rectangular shaped plate of gill I (the plate broadly rounded distally, with divergent or subparallel margins at least throughout of 1/2–2/3 of gill plate length); in contrast to tongue shaped, relatively slender plate of gill I, with margins convergent distally in all other species (cf. e.g. Haybach 1999: 133, fig. 6e; Bauernfeind & Humpesch 2001: 67, fig. 186; Haybach & Thomas 2002: 88, fig. 25; Kłonowska-Olejnik et al. 2007: 58, fig. 17). In both subspecies of E. aurantiacus ( E. aurantiacus aurantiacus and E. aurantiacus androsianus Braasch 1983 ) the gill I is distinctly more slender and smaller compared for example to E. dispar gratificus ssp. nov. (Haybach 1999: 127, fig. 4c; Braasch 1983: 119, fig. 10).
The larvae of E. dispar gratificus ssp. nov. differ from the above listed taxa by the following combination of morphological characters: (i) number of some groups of setae, bristles and hairs on maxillae and labial palpi; the greatest differences were found for: N_CBS (17–22 in E. solus ); N_TCB5 (13–14 in E. belfiorei , 14–17 in E. solus ); N_DOR (14–18 in E. solus ); N_VEN (21–24 in E. solus ); N_PLBas (3 in E. belfiorei , 16–20 in E. solus ); N_PLH (4–5 in E. belfiorei ); N_PLS (up to 25–26 in E. belfiorei ); N_LPH (24–31 in E. belfiorei , 28–36 in E. solus ) (cf. Haybach 1999: 132, 134; Haybach & Thomas 2002: 82, 85; Kłonowska-Olejnik et al. 2007: 57–58); (ii) the shape of pronotal lateral projection (distinctly shorter and rounded apically in E. belfiorei and E. solus , cf. Haybach & Thomas 2002: 86, fig. 20; Kłonowska-Olejnik et al. 2007: 57, fig. 10); (iii) coloration of abdominal terga and sterna (cf. e.g. our Figs. 17–18 View FIGURES 17 – 20 , 21–23 View FIGURES 21 – 30 ; fig. 23 by Haybach & Thomas 2002: 86 showing E. belfiorei ; fig. 15 by Kłonowska-Olejnik et al. 2007: 58 for E. solus ); (iv) shape of scales of dorsal surface of femora (pointed or bluntly pointed spine-like bristles in E. belfiorei , cf. Haybach & Thomas 2002: 86, fig. 21; similar shapes in E. dispar gratificus ssp. nov. and E. solus , cf. Kłonowska-Olejnik et al. 2007: 57, fig. 12); (v) coloration of tarsi (one ring apically in E. belfiorei and E. solus , cf. Haybach & Thomas 2002: 85–86, fig. 22; Kłonowska-Olejnik et al. 2007: 59); (vi) shape of gills (especially gill I) (compare our Figs. 24–30 View FIGURES 21 – 30 and fig. 25 by Haybach & Thomas 2002: 88 for E. belfiorei ; fig. 17 by Kłonowska-Olejnik et al. 2007: 58 for E. solus ).
Using some aspects of the shape and structure of the genitalia, E. dispar gratificus ssp. nov. can be compared primarily with nominal subspecies (see above), and also with some closely related species, e.g. E. aurantiacus , E. belfiorei and E. rothschildi . On the other hand, the genitalia of the new subspecies clearly differ: (i) from E. aurantiacus by the shape of more broadly trapezoidal penis lobes, with distinctly broader apical (rising above the lobes) and lateral sclerites (cf. Thomas 1968: 57, figs. 1, 3; Haybach & Thomas 2002: 82–83, figs. 12–13); (ii) from E. belfiorei by the shape of remarkably wide, straight, not evenly curved apical sclerite (cf. Haybach & Thomas 2002: 81–82, figs. 7–8), and by the presence of distinct borders between basal and lateral sclerites (the same also for E. rothschildi , cf. Thomas & Dakki 1979: 199–200, figs. 8–9); (iii) additionally from E. rothschildi by less massive, not quadrangular penis lobes, and by the presence of more laterally situated small protuberances, directly under the forceps base (cf. our Figs. 9–13 View FIGURES 9 – 13 and fig. 6 by Thomas & Dakki 1979: 199).
The shape of penis lobes of E. dispar gratificus ssp. nov. somewhat resembles those of E. autumnalis , which has been described on the basis of two specimens of male imagoes from the Amtkel River (Western Caucasus Mts.) (Braasch 1980: 103–104, figs. 1 (1a)–2). However, general coloration of body and structure of apical sclerite of penis lobes of E. autumnalis differ considerably from the new subspecies.
Additional differences between E. dispar gratificus ssp. nov. and related taxa are found in the shape and color of the eyes of male imagoes, and partly in color of body and wings. In the male imago of the new subspecies the gap between the eyes is narrower than in E. belfiorei (there is no gap and the eyes touch in the middle in E. aurantiacus and E. rothshildi ). Eyes color of E. dispar gratificus ssp. nov. partly resembles those in E. belfiorei (blue-gray with two violet to dark brown stripes at its base, cf. Haybach & Thomas 2002: 80–81, fig. 2), but mainly without any stripes, only sometimes with one whitish stripe. At the same time eyes color of the new subspecies is different from E. aurantiacus (violet-grey) and E. rothschildi (light gray with one narrow violet-brown (black) ring at the eyes base, cf. Thomas & Dakki 1979: 198).
The arrangement of dark spots on lateral portions of thorax, although is similar in the new subspecies and E. belfiorei , but clearly differs from E. aurantiacus and E. rothschildi . On the other hand, E. belfiorei can be separated from the new subspecies by the presence of a small dark brown patch in each side of posterior margin of terga II −VIII, in addition to brown oblique lateral stripe (Haybach & Thomas 2002: 80–81, fig. 5).
The wings venation of E. dispar gratificus ssp. nov. is generally unicolorous brown, without heavily blackish transversal veins in basal part of forewings as in E. aurantiacus , or violet-black transversal veins in C, Sc and RA area of forewings as in E. belfiorei . In E. rothschildi the transversal veins of forewings are violet-black, black framed in C, Sc and RA area (Thomas & Dakki 1979: 199, fig. 3).
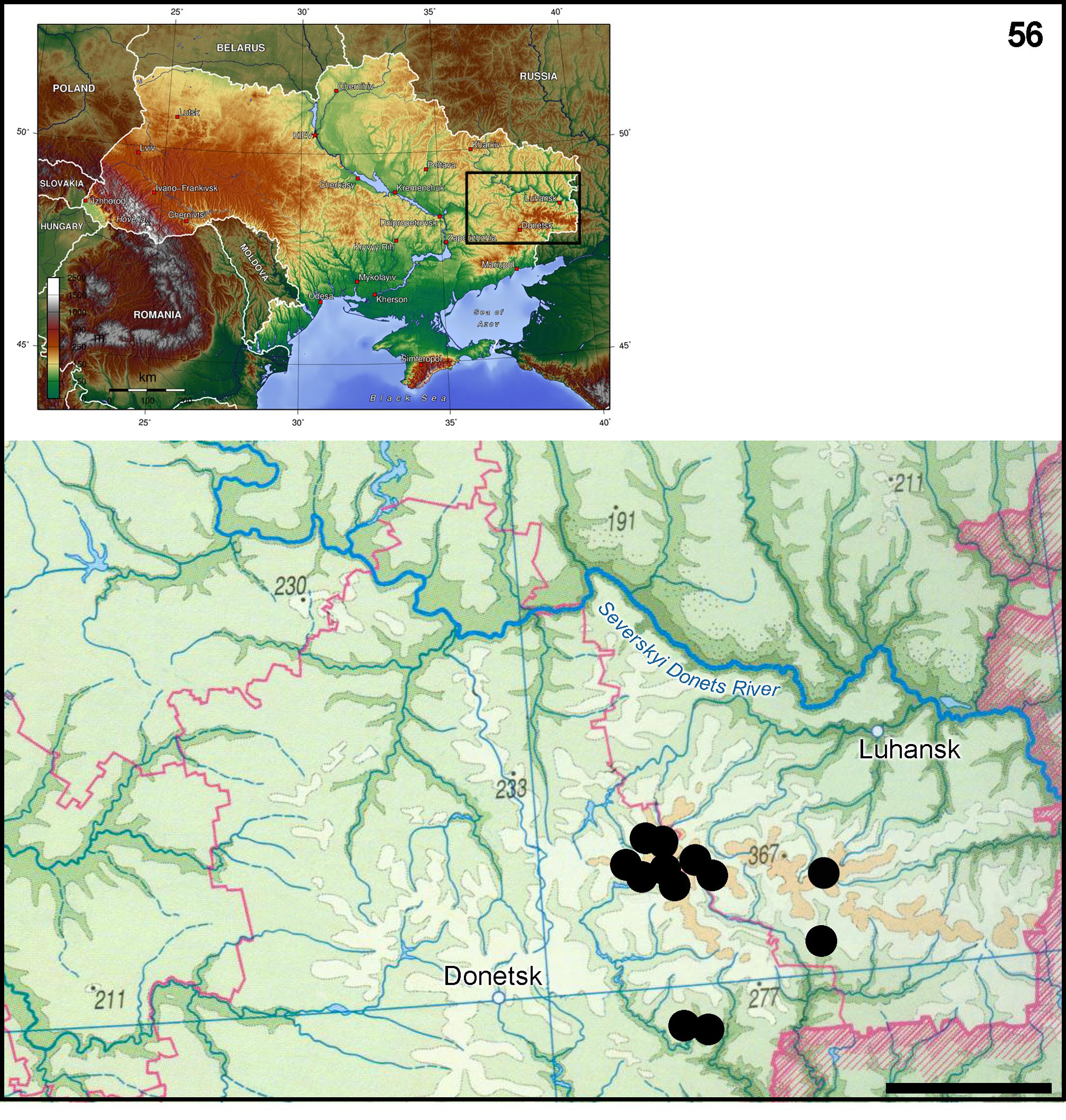
Distribution and biology. Distribution of E. dispar gratificus ssp. nov. is restricted to the Donetsk Elevated area. As known so far, the majority of habitats is located in the highest part of this area within altitudes 250–300 m a.s.l. Analyses of subspecies distribution showed, that its areal is limited to a very small area (about 1.400 square kilometers) ( Fig. 56 View FIGURE 56 ). The distinguishing feature of this area is the presence of dense net of unpolluted rhithral water-flows, covered with woods and running in deep gullies, where shale outcrops appear to be bottom substrate. Such characteristics make water bodies in this area similar to mountain and submountain rivers and streams (Preobrazhenskii 1959) ( Fig. 57–61 View FIGURES 57 – 58 View FIGURES 59 – 61 ).
The subspecies is attached to the rhithral zone, and is found only in river heads and in cold streams. At the moment, the subspecies was registered in the epipotamal water flow only once and in very small number. For this reason the main part of its habitats are aggregated in the highest part of Donetsk Elevated area, which appears to be the watershed between rivers of the Sea of Azov and the Don river-basin (basin of Black Sea). The subspecies is reported mainly from the rhithral zone of streams and rivers located far from this watershed (true altitudes – up to 250 m a.s.l.).
In the type locality (upper part of Bulavina River) larvae of E. dispar gratificus ssp. nov. grow at annual temperature variation from 3.2 ºС to 18ºС.
All sections of water-flows, which are populated by the subspecies, flow in deep gullies overgrown with forest. Their riverbeds appear to be in the shadow of trees along the whole length ( Fig. 57−61 View FIGURES 57 – 58 View FIGURES 59 – 61 ).
It should be noted, that E. dispar dispar , in spite of E. dispar gratificus ssp. nov., is characterized by much larger ecological valence, and is able to develop not only in rhithral and epipotamal, but also in hypopotamal water bodies (rivers, streams), and even in channels and lakes (Yaroshevskyi, 1881; Fadeev, 1929; Solodovnikov, 1940; Sartori & Landolt, 1999; Soldán & Zahrádková, 2000).
Like all other representatives of the genus, E. dispar gratificus ssp. nov. is a typical lithoreophile. The majority of new subspecies larvae are found on shallows, stony river bed (with large or small stones) and on sunk logs in river sections with current, where they present the base of number of all mayflies in summer time. The subspecies is found both in rivers up to 10 m wide, and in streams more than 1 m wide and from 0.1 m deep ( Fig. 61 View FIGURES 59 – 61 ).
The larvae were recorded under current velocity ranging from 0.2 to 1 m /sec. The highest population density (47 ind/m2) was observed on the large-stone sections of river bed up to 0.4 m deep and with current velocity of 0.2−0.4 m /sec.
The larvae of different size classes occur all over the year. Mature larvae, imagoes and subimagoes could be found from late May to the middle of September. The highest number of nymphs has been observed from late July to late August.
According to the classification of life-cycles by Clifford (1982), E. dispar gratificus ssp. nov. exhibits Uw − Us cycle (a seasonal univoltine cycle where most of the new generation overwinters in the egg stage but a small part of the population overwinters as larvae).
E. dispar dispar demonstrates mainly the Us cycle within Europe ( Landa, 1968; Haybach, 1998; Soldán & Zahrádková, 2000). Some authors suppose that this subspecies has the Uw, Uw − Us and bivoltine life cycles (Macan & Maudsey, 1968; Sowa, 1975; Elliott et al., 1988; Humpesch, 1981).
It should be noted, that larvae of E. dispar gratificus ssp. nov. are rather susceptible to pollution and to changes in temperature conditions. Thus, in Bulavina River the subspecies is found only in its head, before a large stream inflow (it runs out of the city sedimentation basin and has a large pond 300 m before its inflow into Bulavina River). Downstream from the confluence the water in the river is more turbid and the bed is silted, its temperature conditions correspond to potamal zone. E. dispar gratificus ssp. nov. larvae are found only in a small quantity in this part of the river; their presence here could be explained by the drift.
There are no larvae of E. dispar gratificus ssp. nov. in the river 5–6 km lower from the inflow of a big stream, where the family Heptageniidae is presented only by the species Heptagenia flava Rostock, 1878 , which is not found in the river head.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |