Dipteropeltis campanaformis, Neethling, Lourelle Alicia Martins, Malta, José Celso De Oliveira & Avenant-Oldewage, Annemariè, 2014
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3755.2.4 |
|
publication LSID |
lsid:zoobank.org:pub:44846DB8-02B4-4806-9B11-CAD57E716650 |
|
DOI |
https://doi.org/10.5281/zenodo.6143745 |
|
persistent identifier |
https://treatment.plazi.org/id/03DBB164-FFD2-8C5C-0191-E70A0CE3F25F |
|
treatment provided by |
Plazi |
|
scientific name |
Dipteropeltis campanaformis |
| status |
sp. nov. |
Dipteropeltis campanaformis n. sp.
( Figure 3 View FIGURE 3 B, 4–6)
Etymology. The species name campanaformis refers to the bell shape that the carapace lobes resemble.
Type locality. In an unnamed forest stream (”Igarapé”, Água Branca) in Reserva Florestal Ducke, in the Amazonas River basin in Manaus, Amazonas, Brazil.
Type host. Brycon amazonicus (Spix and Agassiz, 1829)
Material examined. Holotype. Egg-bearing ♀ ( INPA1934 , former INPA 423iii, see Table 1) here designated . Paratypes. 6 egg-bearing ♀: 4 egg-bearing ♀ ( INPA 423 i, ii, iv, v, see Table 1) collected from Brycon amazonicus (Spix and Agassiz, 1829) from an unnamed forest stream (”Igarapé”, Água Branca) in Reserva Florestal Ducke , in the Amazonas River basin in Manaus, Amazonas, Brazil, during July 1983 ; 2 egg-bearing ♀ ( INPA 1755i , ii, see Table 1) collected from an Acestrorhynchus sp .( Acestrorhynchidae ) from the Padauari River, a left bank tributary of the upper Negro River , Amazonas, Brazil during October 1981 .
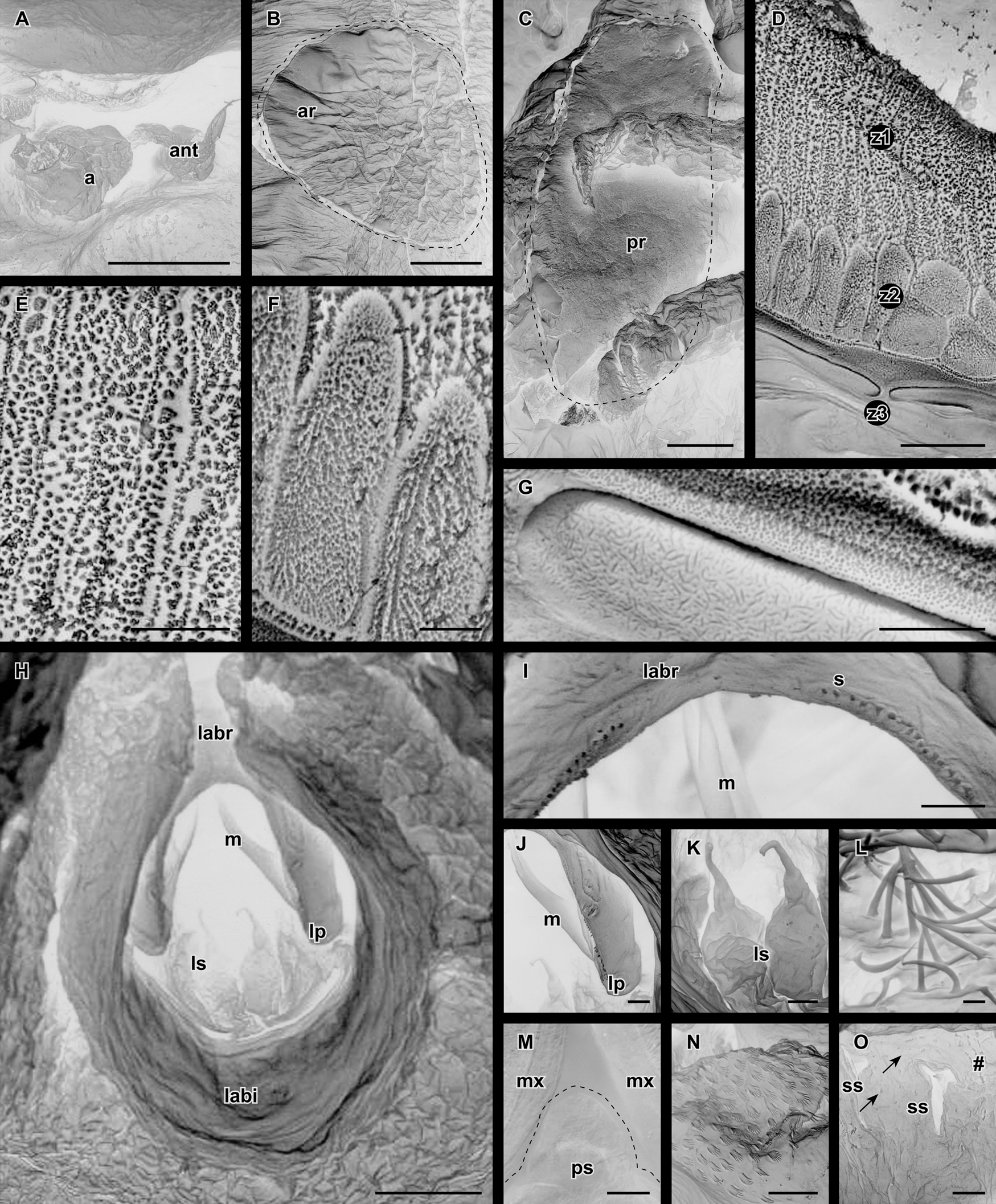
Description. In dorsal view ( Fig. 4 View FIGURE 4 A), this species has a narrow, square-shaped head shield that does not have folds nor does its width exceed the outer margins of the maxillules. Two compound eyes (ce) are visible. A pair of separate inter-ocular rods (ir) originates between the eyes, extends towards the nauplius eye (ne) and continues around it. The carapace lobes (HC, Fig. 3 View FIGURE 3 B, 4A–B, Table 2) are 13 mm long (range 8–16 mm, average 12.7 mm) with the split length of the carapace (CSL) at 78.46 % of the length of the carapace (CSL/HC, average 78.55 %, Table 2). The elliptical lobes give the parasite a bell shape and the lobes only shield the anterior part of the abdomen. The central duct of the midgut on the central axis of the lobes is visible in light microscopy with a network of tubules branching out.
In ventral view ( Fig. 3 View FIGURE 3 B, 4B); the head shield is cucullate and visible above the anterior margin of the maxillules (mx). There are two respiratory areas on the ventral side of each carapace lobe visible in scanning electron microscopy; the anterior area (ar, Fig. 4 View FIGURE 4 B, 6B) is a small (0.27 mm x 0.35 mm) triangular oval, while the posterior area (pr, Fig. 4 View FIGURE 4 B, 6C) is large and oval (1.31 mm x 2.97 mm). There are no hooks or spines. The total length of the specimen (HA) is 15 mm, with range between 10.00–20.00 mm (average 15.14 mm, see Table 2).
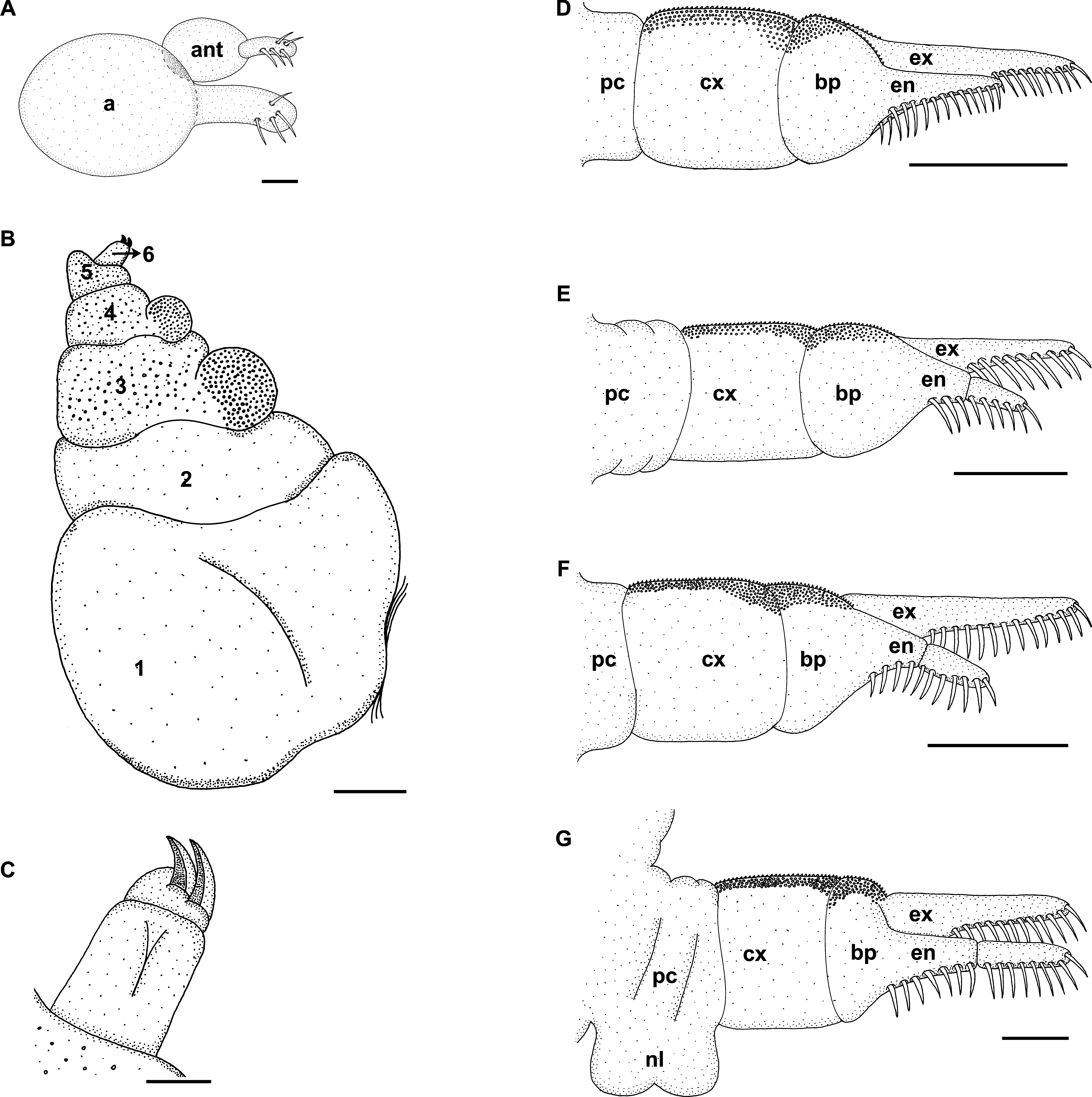
The antennules and antennae ( Fig. 5 View FIGURE 5 A, 6A) are obscured by the suckers. The antennule (ant) is small (64 Μm) and two segmented; the base is narrow and oval, the terminal segment cylindrical, with a blunt tip that carries five setae. The antenna (a, 135 Μm) is two segmented; the base is bulbous and oval; the terminal segment cylindrical, carrying four setae.
The maxillules (mx, Fig. 4 View FIGURE 4 B, 6D) are cylindrical stalks with oval membranous suction discs that lack supportive rods (characteristic of Argulus and Chonopeltis ). The cup rim is divided into three zones ( Fig. 6 View FIGURE 6 D); zone 1 (interior margin) bears prominent radiating rows of micro papillae ( Fig. 6 View FIGURE 6 E); zone 2 (middle) with suctorial plates ( Fig. 6 View FIGURE 6 F); zone 3 (exterior margin) with two rows of concentric elongated discoidal scales ( Fig. 6 View FIGURE 6 G). The sucker diameter (SD, Table 2) is 0.70 mm and constitutes a ratio of 0.05 (4.67 %) of the total length (SD/HA) of the specimen (range 0.03–0.07 mm, average 5.0 %).
In the mouth (mo, Fig. 4 View FIGURE 4 B, 6H), the labrum (labr, upper lip) is an inverted U-shape with lateral protrusions (lp) and a single row of short setules (s, Fig. 6 View FIGURE 6 I). The lateral protrusions form the base of the mandibles (m) which are sickle-shaped with 3 or 4 slim and sharp denticles at the distal end ( Fig. 6 View FIGURE 6 J). The labium (labi, lower lip) encircles the upper lip, making the mouth opening circular in shape. There are two short tubular labial spines (ls, 54 Μm) at the entrance to the mouth ( Fig. 6 View FIGURE 6 K). The internal dorsal surface of the mouth is densely packed with long setae ( Fig. 6 View FIGURE 6 L). The pre-oral structure (ps) is diminutive and triangular in shape, without a duct or spine ( Fig. 6 View FIGURE 6 M).
The maxillae (ma, Fig. 4 View FIGURE 4 B, 5B) are prominent, conical, and directed vertically, forming a cubic shape in the head region. It is six-segmented; with a wide (segment 1, 727 Μm) base, tapering to the distal segment. Segments three and four bear round protrusions on their medial surfaces with pectinate scales ( Fig. 6 View FIGURE 6 N). Segment six bears two stout setule-like claws ( Fig. 5 View FIGURE 5 C). All the segments are sparsely covered by setae.
The swimming legs are biramous. Leg 1 ( Fig. 5 View FIGURE 5 D), the precoxa (pc) is short (200 Μm), the coxa (cx) longer (488 Μm), the basopodite (bp, 250 Μm) bears the endopodite (en, 350 Μm) and the exopodite (ex, 600 Μm). Leg 2 ( Fig. 5 View FIGURE 5 E), the precoxa (pc) is short (166 Μm), the coxa (cx) longer (452 Μm), the basopodite (bp, 286 Μm) bears the two-segmented endopodite (en, 242 Μm) and the exopodite (ex, 362 Μm). Leg 3 ( Fig. 5 View FIGURE 5 F), the precoxa (pc) is short (182 Μm), the coxa (cx) is longer (484 Μm), the basopodite (bp. 348 Μm) bears the two-segmented endopodite (en, 302 Μm) and the exopodite (ex, 664 Μm). Leg 4 ( Fig. 5 View FIGURE 5 G), the precoxa (pc, 384 Μm) bears the bilobed natatory lobe (nl, 203 µm) on the posterior surface, the coxa (cx) is longer (400 Μm); the basopodite (bp, 150 Μm) bears the twosegmented endopodite (en, 566 Μm) and the exopodite (ex, 566 Μm). All 4 legs bear round scales on the coxa and basopodite, with a denser concentration on the anterior surface.
The abdomen ( Fig. 3 View FIGURE 3 B, 4A, 4B) has an angular base. Lobes (AL, Table 2) are 7 mm long (range 5–10 mm, average 7.21 mm) and lanceolate, with tips that bend slightly inwards. The abdomen has a ratio of 0.47 (46.67 %) of the length of the specimen (AL/HA, Table 2) with the species average at 47.80 %. Furcal rami were not observed at the position of the split. The average abdominal sinus (ASL) is 86.14 % of the length of the abdomen (ASL/AL, see Table 2). The spermathecae (sp, Fig. 4 View FIGURE 4 B) are oval with angular anterior and posterior apices; the area is flattened and sparsely scattered with stout pectinate scales interspersed with setules ( Fig. 6 View FIGURE 6 O).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |