Arcynopteryx dichroa ( McLachlan, 1872 )
|
publication ID |
https://doi.org/ 10.5281/zenodo.210960 |
|
DOI |
https://doi.org/10.5281/zenodo.6174154 |
|
persistent identifier |
https://treatment.plazi.org/id/8E1087A9-FFF0-8978-FF2D-1EF8A301F9F6 |
|
treatment provided by |
Plazi |
|
scientific name |
Arcynopteryx dichroa ( McLachlan, 1872 ) |
| status |
|
Arcynopteryx dichroa ( McLachlan, 1872) View in CoL
( Figs 1–12 View FIGURES 1 – 6 View FIGURES 7 – 8 View FIGURES 9 – 12 )
McLachlan 1872: 52−53, pl. I, figs 4−4a, 5−5b ( Dictyopteryx dichroa ); Klapálek 1912: 14–16, fig. 8 ( Arcynopteryx compacta ); Brinck 1949: 58−60, figs 4 A–F ( Arcynopteryx compacta ); Brinck 1956: 66–71, figs 6A–D ( Arcynopteryx compacta ); Zhiltzova 1966: 539 ( Arcynopteryx compacta ); Illies 1966: 353 ( Arcynopteryx dichroa ); Kimmins 1970: 340 ( Arcynopteryx dichroa ); Zwick 1973: 224 ( Arcynopteryx compacta ); Stark & Szczytko 1988: 156 ( Arcynopteryx compacta ); Kondratieff 2004: 166, figs 8.1–8.3 ( Arcynopteryx compacta ); Stewart & Oswood 2006: 182 ( Arcynopteryx compacta ); Teslenko & Zhiltzova 2009: 26, figs. 118−121 ( Arcynopteryx compacta ). The additional synonyms are presented in Brinck’s (1949), Ricker’s (1952), Illies’s (1955) papers, on the Plecoptera species file website (DeWalt, Neu-Becker & Stueber 2012).
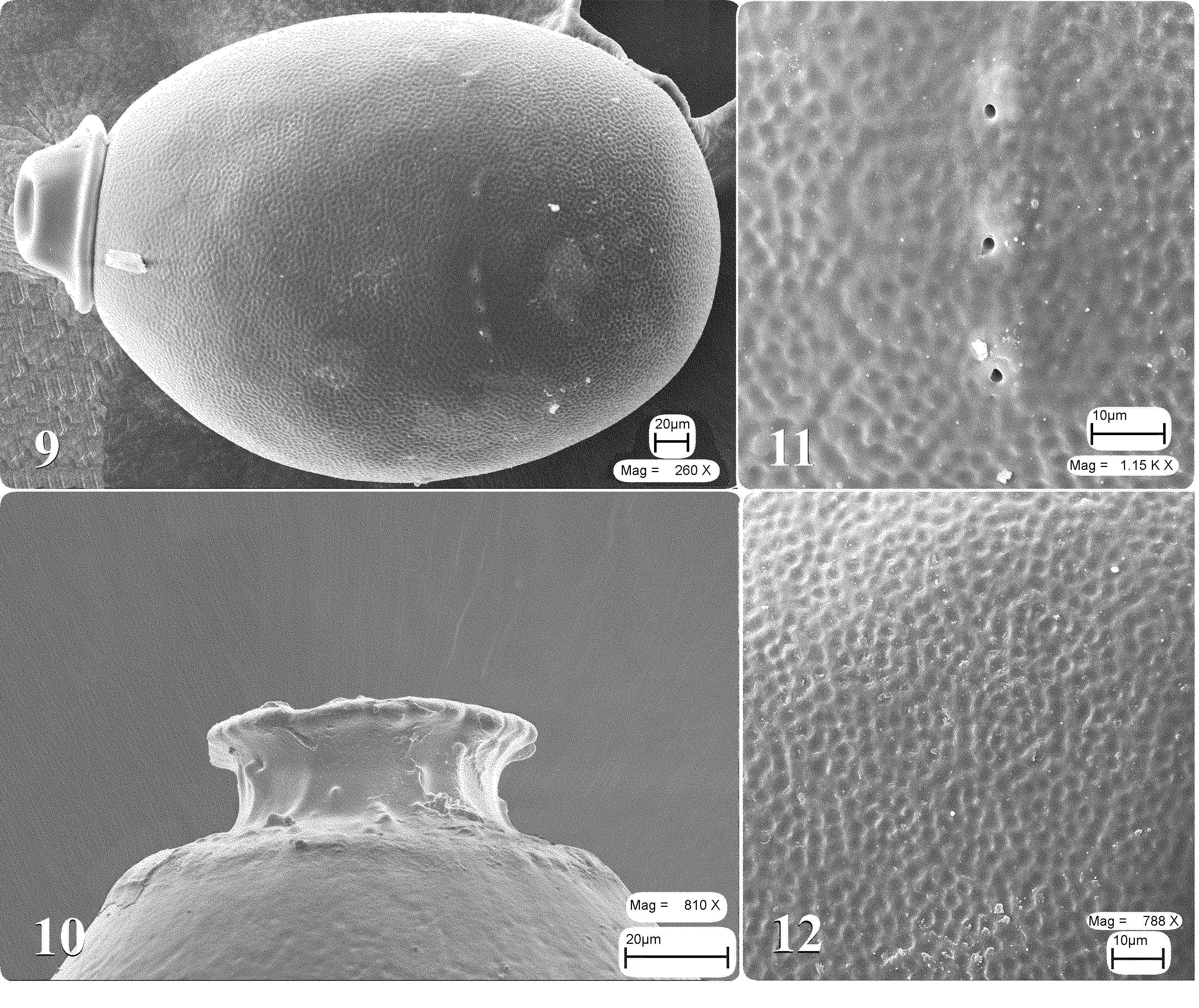
Diagnosis. A. dichroa can be distinguished by a shape of the hemitergal lobes which bear lobe apices directed posteriorly, apical margins prolonged and rounded, well sclerotized ( Fig. 3 View FIGURES 1 – 6 ); the hemitergal lobes are in contact mesoanteriorly ( Figs 2 View FIGURES 1 – 6 & 7 View FIGURES 7 – 8 ). Each hemitergal lobe bears a knob erected above a membranous patch close to the anterior hemitergal margin ( Figs 2, 3 View FIGURES 1 – 6 & 8 View FIGURES 7 – 8 ). Epiproct is very distinct from that of the other Arcynopteryx species, the stylet of the epiproct resembles a strong, long, fine bristle directed upward and forward ( Fig. 5 View FIGURES 1 – 6 ). The shape of female subgenital plate is variable: the posterior margin of the subgenital plate has a shallow notch that separates two small lobes ( Fig. 6 View FIGURES 1 – 6 ); sometimes there are two shallow notches with three small lobes. Egg is distinguished by chorionic structure which is presented by a pattern of hexagonal follicle cell impressions (FCI’s), flat floors which contain often 12 shallow punctations ( Fig. 12 View FIGURES 9 – 12 ).
Adult habitus. The head, pronotum and abdomen are brown, the meso- and metanotum dark brown. Head ( Fig. 1 View FIGURES 1 – 6 ) with wide, transverse, M-shaped dark brown band between antennal bases, delimited by epicranial suture posteriorly. In front of the distinct M-shaped band, an indistinct darkish spot projects onto the clypeus; the frontoclypeus is broadly truncated and pale laterally; the tentorial pits are dark ( Fig. 1 View FIGURES 1 – 6 ). The interocellar area exhibits a pale spot rounded anteriorly that does not reach the median ocellus, a pale spot continues to the medial surface of the occiput. Two tentorial pits in front of the lateral ocelli and two small oval patches laterally to the lateral ocelli are pale. Behind each compound eye is a posterolateral spot with brown callosities ( Fig. 1 View FIGURES 1 – 6 ). The antennae and palpi are brownish; the basal antennal segments are brown. The submental gills are long and thin. The pronotum is the same width as the head width under the compound eyes, brownish, quadrangular, with rounded angles; the lateral margins are straight; a broad, median yellow band is present, slightly wider in its posterior third ( Fig. 1 View FIGURES 1 – 6 ). The pronotal rugosities are dark brown. The lateral fields are slightly darker than the median stripe, and the pronotum callosities are sometimes indistinct. The arms of the mesosternal ridge meet the anterior corners of the furcal pits. The abdomen is covered by colorless hairs, pronounced on the abdominal terga posterolaterally. The legs are brown; the distal end of the femur and the basal part of the tibia are dark brown. The cerci are longer than the abdomen, with long brownish hairs; the basal cercal segments are brown. The distal half of the apical cercal segments is dark brown. The females are macropterous. The males usually have very shortened wings, or their wings reach to the end of the abdomen or slightly past it. The forewing is long, narrow, and transparent, with brown veins and a pale yellow C vein. The venation includes an irregular net near the apex, sometimes consisting of three rows of cells. The hind wing anal area is large, and A2, A4 and A5 are forked. Brachypterous and long-winged specimens occurred together at the same sampling site.
Male. Body length 10.2−19.5 mm, forewing of full-winged male 12.0− 12.7 mm, wingspan 25.4–27.0 mm; forewing of male with shortened wings 4.3−6.0 mm, wingspan 10.1–13.5 mm. Abdominal tergum 9 exhibits a thin, transversal, membranous, pale median line and two pairs of small pale spots: one pair of spots is close to the line medially; the second pair of spots is situated close to the anterior margin of tergum 9 ( Fig. 2 View FIGURES 1 – 6 ). The posterior margin has a medial arcuate notch, which runs ½ of the length of tergum 9, and two submedial, transversely elongated, and rounded swellings. These swellings are covered by small stout setae close to the notch and by long fine colorless hairs posteriolaterally ( Figs 2 View FIGURES 1 – 6 & 7 View FIGURES 7 – 8 ). Sternum 9 is scoop-shaped, extended backward and curved upward. Tergum 10 is divided into two hemiterga ( Fig. 2 View FIGURES 1 – 6 ). The hemitergal lobes in a dorsal view are wide, with lobe apices directed posteriorly, apical margins prolonged and rounded, well sclerotized; the lobes are in contact mesoanteriorly ( Figs 2, 3 View FIGURES 1 – 6 & 8 View FIGURES 7 – 8 ). Their mesal edges are membranous and resemble prolonged small white oval patches. Each hemitergal lobe bears a knob erected above a membranous patch close to the anterior hemitergal margin ( Figs 2 View FIGURES 1 – 6 & 8 View FIGURES 7 – 8 ). The knob is ovular, membranous ventrally, sclerotized and rounded dorsally, and covered by a few small, stout setae ( Figs 3 View FIGURES 1 – 6 & 8 View FIGURES 7 – 8 ). The cowl is membranous, folded, resembles a deep pouch between and under the hemitergal lobes and is attached around the base of the epiproct and the internal basal anchor ( Figs 5 View FIGURES 1 – 6 & 8 View FIGURES 7 – 8 ). The dorsolateral edges of the cowl are supported by flat and darkly sclerotized paragenital plates. Otherwise, in a lateral view, the lever arm of the epiproct is arcuate and is found at the bottom of the deep cowl; and terminates in a basal sclerite ventrally, the top of which serves as a place for the attachment of the loop of the stylet and sclerotized bands ( Fig. 5 View FIGURES 1 – 6 ). The stylet of the epiproct resembles a strong, long, fine bristle directed upward and forward; the basal plate of the loop of the stylet is thin ( Fig. 5 View FIGURES 1 – 6 ). Two lateral sclerotized bands are narrowed at the base, and each band has a deep rounded notch on the inner edge in the last third of its length. In a dorsal view, the everted aedeagus is large and membranous, with a pair of lateral lobes at dorsolateral margins ( Fig. 4 View FIGURES 1 – 6 ). The lobes may not be fully everted. One large prolonged lobe is narrowed to the apex. Fine, erect, clear spinules are visible ventrally and dorsally on the prolonged lobe. The swollen rounded apex of the aedeagus is without spinules ( Fig. 4 View FIGURES 1 – 6 ).
Female is macropterous, larger and darker than male. Body length 11.0− 20.5 mm, forewing 11.0– 18.4 mm, wingspan 24.0− 39.2 mm. Sternum 8 bears two brown spots close to the anterior margin ( Fig. 6 View FIGURES 1 – 6 ). The subgenital plate is wide, relatively short, and pale mesoanteriorly, extending laterally from the sides of sternum 8 and reaching almost half the length of sternum 9 ( Fig. 6 View FIGURES 1 – 6 ). The posterior margin of the subgenital plate has a shallow notch that separates two small lobes. The subgenital plate covered with small, brownish setae. The shape of the subgenital plate is variable. Sometimes there are two shallow notches with three small lobes. Sternum 9 is pale medially, with two brown circular spots mesolaterally ( Fig. 6 View FIGURES 1 – 6 ).
Egg is ovular and circular in cross-section, 370 X 238 µm. Anchor mushroom-shaped covers the collar completely ( Fig. 9 View FIGURES 9 – 12 ). The collar is stalked, its rim flanged and irregularly incised ( Fig. 10 View FIGURES 9 – 12 ). The sides of collar has irregular meshwork and projections; shoulder is low ( Fig. 10 View FIGURES 9 – 12 ). The chorion is covered with hexagonal FCI’s ( Figs 9 & 12 View FIGURES 9 – 12 ); the FCI walls are slightly raised with thin, shallow furrows; flat floors often contain 12 shallow punctations ( Fig. 12 View FIGURES 9 – 12 ). Row of micropyles subequatorial; orifices small without lips, some set on low micropylar mounds are occasionally surrounded by rosettes ( Figs 9 & 11 View FIGURES 9 – 12 ). Eclosion line is absent.
Material examined. Lectotype male (pinned), D. dichroa McLachlan (Dictyopteryx) , [McL. label] / Sibir. orient. (Maa(c)k) / dichroa McL. / Dictiopteryx dichroa McL. The type-series in BMNH includes one male and one female paralectotypes ( Kimmins 1970). Russia: Altai Mountains, 12 males, 2 females, Chuya River, near Iodro settlement, 18.05.1989, coll. E. Makarchenko; Khabarovskiy Region, 10 males, 2 females, Khor River, Ussuri R. Basin, Amur R. Basin, 31.05.1961, coll. I. Levanidova; Tuva: 3 males, 2 females, Kara-Hol‘ Lake, Baj-Taiginskyi District, 23.06.2003, coll. M. Zasypkina; Kamchatka Peninsula, 6 males, 3 females, Avacha R., below Krutaya R. mouth, 20.06.1969, coll. I. Levanidova; Chukotka Peninsula, 1 male, Levaya Rechka, 105 km on the road between Egvekinot and Iultin settlements, 3.07.1973, coll. I. Chereshnev; 1 male, 1 female, Pravaya Rechka, 105 km on the road between Egvekinot and Iultin settlements, 10.07.1973, coll. I. Chereshnev; 1 male, Lena River, Lenskie Stolby, 1.06.1974, coll. I. Levanidova.
Distribution. A. dichroa , is a Holarctic circumpolar species primarily of the northern latitudes of Europe, Asia, and North America, occurring in streams, but also lakes throughout its range. The species inhabits northern Europe ( Zhiltzova 1966, Lillehammer 1974, Loskutova 2006), in Central and Southwest Europe A. dichroa has a disjunct boreomontane distribution with Pleistocene relict populations ( Illies 1955, Zwick 2004). The species is widespread in Asia including Mongolia, Siberia and the Russian Far East. In North America A. dichroa also occurs in alpine zones of Central Rocky Mountains ( Ricker 1964, Stewart & Oswood 2006). Populations of A. dichroa have been recorded from Alaska, Alberta, British Columbia, Montana, Colorado, Maine, New Hampshire, Saskatchewan and Wyoming ( Stewart & Stark 2002).
Remarks. Arcynopteryx dichroa has been described based on male and female specimens as Dictyopteryx dichroa ( McLachlan 1872) . The description has omitted the date, locality and the structural details of the epiproct complex. However, the stylet of the epiproct was noted and illustrated ( McLachlan 1872, pl. I. Fig. 4 View FIGURES 1 – 6 ). Dictyopteryx dichroa type is well associated with newly acquired material in the shape of the hemitergal lobes and knobs on them. These characters suggest that the species D. dichroa belongs to Arcynopteryx Klapálek, 1904 with the name Arcynopteryx dichroa ( McLachlan, 1872) .
Klapalek designated Arcynopteryx compacta ( McLachlan, 1872) as type species of genus Arcynopteryx . However, he was mistaken and misidentified, when provided ( Klapálek 1912, fig. 8, page 13) an illustration of “ Arcynopteryx compacta McLachlan ”, which was actually A. dichroa (McLachlan) , thus both taxonomic species actually were involved in the misidentification (International Code of Zoological Nomenclature 1999). The type species of Arcynopteryx is fixed (under Article 70.3.2 of the Code) as Arcynopteryx dichroa ( McLachlan, 1872) , misidentified as Arcynopteryx compacta ( McLachlan, 1872) in the original designation by Klapalek (1912).
The shape of the female subgenital plate of Arcynopteryx dichroa is variable ( Brinck 1949, Illies 1955, Zhiltsova 1964, Rauser 1968). In the East Palaearctic and Nearctic Regions the females without associated males are difficult to separate from those of Skwala or A. polaris , since their subgenital pates are similar, and both genera have the arms of mesosternal ridge meet anterior corners of furcal pits ( Stewart & Oswood 2006).
Wide variations in body size, wing length and color pattern are observed throughout the distribution of the species ( Illies 1955, Lillehammer, 1988). For example, Despax (1951) described three forms of the species with different wing lengths from the Pyrenees. In the population from Kara-Hol Lake in Asia (Tuva, Siberia, Russia), the head and pronotum of males are generally pale, with an indistinct brownish pattern. On the Chukotian and Kamchatka peninsulas (the Far East of Russia), the stoneflies have a dark brown body pattern, and the males are usually brachypterous. Both the males and the females from the Ussuri River (Amur R. Basin) have long wings ( Levanidova & Zhiltzova 1979).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |