Dactylogyrus skrjabini Achmerow, 1954
|
publication ID |
https://doi.org/ 10.12782/specdiv.25.61 |
|
DOI |
https://doi.org/10.5281/zenodo.4734633 |
|
persistent identifier |
https://treatment.plazi.org/id/357D87B8-9A54-9571-FC3A-F9DEFA889270 |
|
treatment provided by |
Felipe |
|
scientific name |
Dactylogyrus skrjabini Achmerow, 1954 |
| status |
|
Dactylogyrus skrjabini Achmerow, 1954 View in CoL
[New Japanese name: Dai-yubigata-mushi]
( Fig. 2 View Fig )
Dactylogyrus skrjabini Achmerow, 1954: 167–168 View in CoL , fig. 1; Bogdanova 1957: 1391–1393; Long and Lee 1960: 218–219, fig. 2; Akmetov 1963: 462; Lee 1963: 76; Yamaguti 1963a: 30; Musselius 1969: 237–238, 240; Bauer and Hoffman 1976: 165; Gvozdev and Agapova 1977: 109; Rohde 1979: 655; Hoffman and Schubert 1984: 238; Ali et al. 1989: 152–153; Gibson et al. 1996: 29; Blanc 1997: 497; Xia et al. 2000: 152; Grigorovich et al. 2002: 1208; Johnson and Lunde 2005: 132; Al-Saadi et al. 2010: 3, 4; Karabekova 2008: 331, 333; Mhaisen et al. 2012: 107, 116; Zhang 2012: 123; Al-Jawda and Asmar 2015: 129; Mhaisen and Al-Rubaie 2016: 5, 7.
Copepods were removed from the gills using small needles and forceps and fixed in 70 or 99% ethanol. Copepods were cleared and dissected in lactic acid. The whole body was examined using the wooden slide method ( Humes and Gooding 1964). The removed appendages and parts of the body were dehydrated through a graded ethanol series, cleared in xylene, mounted in Canada balsam, and examined for morphological characters.
Drawings were made with the aid of a drawing tube fitted on an Olympus BX51 light microscope. Measurements, in micrometers, are expressed as the range. The monogenean and copepod specimens are deposited in the Platyhelminthes and Crustacea collections of the National Museum of Nature and Science (NSMT-Pl and NSMT-Cr), Tsukuba City, Ibaraki Prefecture, Japan, respectively.
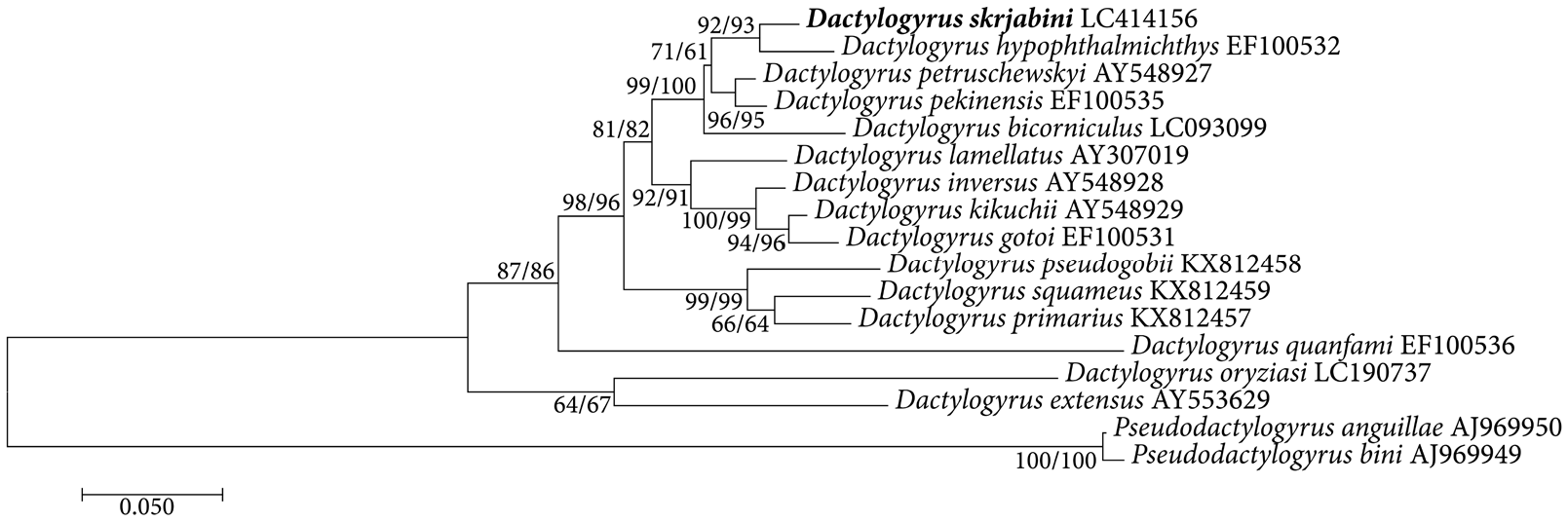
DNA was extracted from two specimens of D. skrjabini View in CoL using the DNeasy blood and tissue kit (Qiagen) in accordance with the manufacturer’s instructions. The DNA was amplified by polymerase chain reaction (PCR) using the primer pair C1 (5′ -ACC CGC TGA ATT TAA GCA T- 3′) and D2 (5′ -TGG TCC GTG TTT CAA GAC- 3′) to amplify partial 28S rDNA ( Vân Le et al. 1993). A total of 25 µL PCR reaction consisted of 1 µL of DNA template, 10×Titanium Taq PCR Buffer (Clonetech), 0.2 mM of each dNTP, 1 μ M of each primer, and 1×Titanium Taq DNA Polymerase (Clonetech). PCR was carried out with the following protocol: 94°C for 5 min followed by 35 cycles of 94°C for 60 sec, 56°C for 60 sec and 72°C for 60 sec, and 5 min of final hold at 72°C. PCR product was purified using NucleoSpin Gel and PCR Clean-up kit (Macherey-Nagel) and sequenced with a 3130xl Genetic Analyzer (Applied Biosystems) with the same primers that generated the PCR product. The newly generated 28S rDNA sequence was aligned with sequences for 14 Dactylogyrus View in CoL species and two Pseudodactylogyrus View in CoL species collected in East Asia retrieved from the GenBank database ( Fig. 3 View Fig ). Alignment was performed with ClustalW using the default parameters. Phylogenic trees were constructed for maximum likelihood methods under the GTR+G+I model selected as the best-fit model using AICc, and with the neighbor-joining (NJ) method under the K2 model, with the phylogeny tested by 1,000 bootstrap repeats using MEGA7 ( Kumar et al. 2016).
Dactylogyrus scrjabini View in CoL [lapsus]: Bykhovskaya-Pavlovskaya et al. 1962: 254–255, fig. 603; Babayev 1964: 51; Gussev 1967: 56, 58, figs 1ge, 2ze; Osmanov 1971: 104; Yukhimenko 1972: 155, 156; Anonymous 1973a: 139, pl. 78, figs 157–158; Musselius 1973: 20–21, fig. 6be; Anonymous 1978: 50; Chen 1981: 115; Ji et al. 1982: 20; Molnár 1984: 154; Gussev 1985: 22, 122–123, figs 9-8, 157; Huang 1986: 16; Salih et al. 1988: 371, 378, 381–382, fig. 7; Gerasev 1989: 39–40, fig.1-1; Gerasev 1990: 367; Gerasev 1991: 224–226, fig. 5-18; Hoffman 1999: 128; Urazbaev and Kurbanova 2006: 537; Long 2000: 95–96, fig. 42; Zonn et al. 2009: 125; Gussev et al. 2010: 23, 187–188, figs 4-8, 213; Zhao 2011: 22–23, fig. 2-10; Davydov et al. 2012: 141.
Material examined. Five specimens stained in alum carmine and three fixed in modified picrate glycerin (NSMT-Pl 6393).
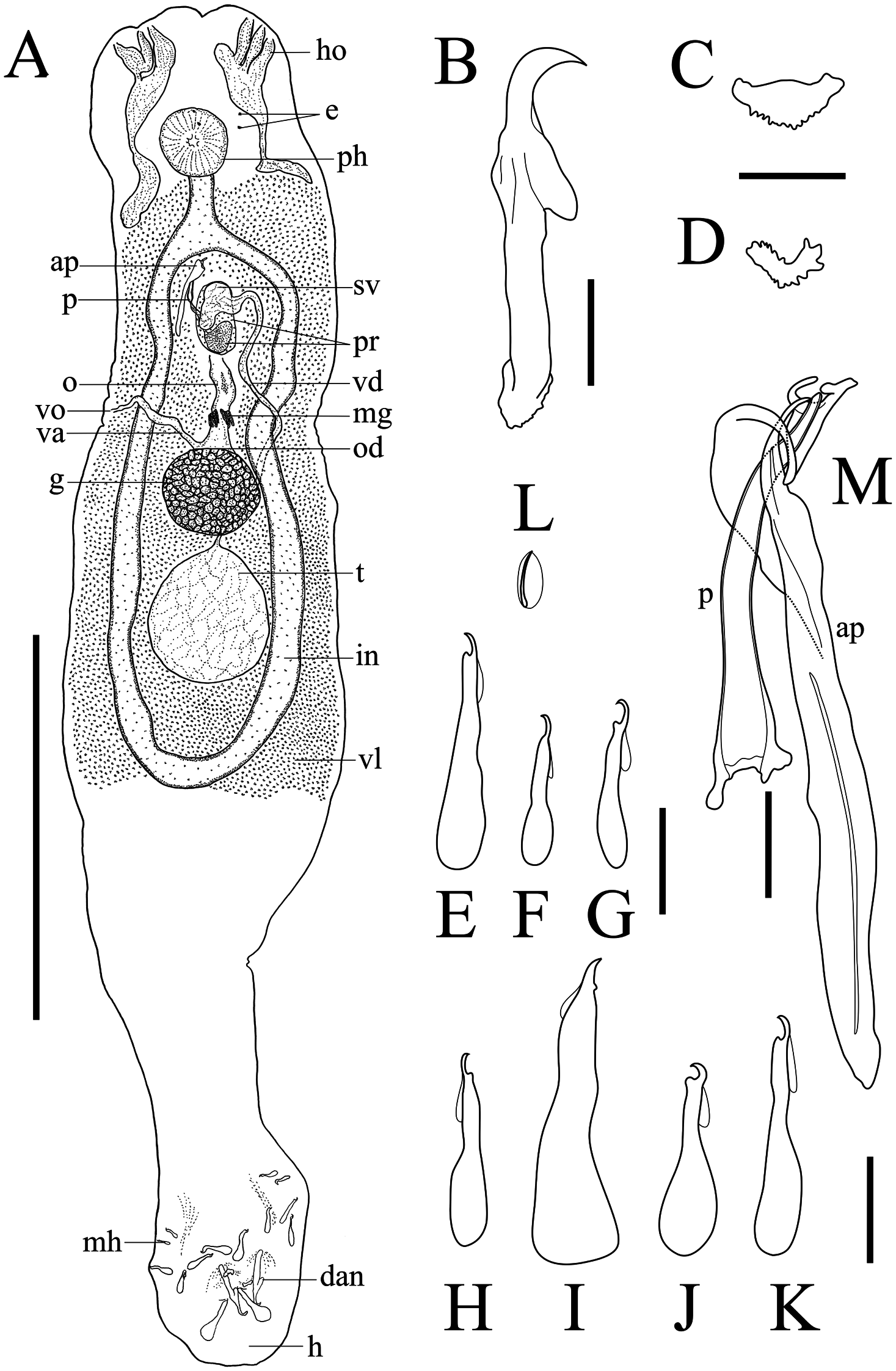
Description. Body elongate ( Fig. 2A View Fig ), 1208–2618 long including haptor and long peduncle, width at mid-body. Three pairs of head organs. Two pairs of eye-spots. Pharynx subspherical, 80–103 long, 82–104 wide; esophagus followed by bifurcated intestine with branches confluenting posterior to testis. Testis ovate to pyriform, posterodorsal to germarium, 128–298 long, 115–158 wide. Vas deferens arising from anterior end of testis, looping dorsoventrally around left intestine, forming seminal vesicle. Two saccate prostatic reservoirs. Male copulatory organ sclerotized, consisting of penis and accessory piece, length 104–128 ( Fig. 2M View Fig ). Penis slightly curved tube, length 68–84. Accessory piece rod-shaped, its widened tip holding distal end of penis, length 104–127. Germarium ovate, in mid-body, 60–229 long, 82–120 wide. Oviduct arising from anterior margin of germarium, continuing to oötype. Mehlis’ gland surrounds base of oötype. Vagina unsclerotized, opening on right lateral side, midlength of body, leading to right side of oviduct. Vitellaria approximately co-extensive with intestine.
Haptor 185–250 long, 180–250 wide. Dorsal anchor ( Fig. 2B View Fig ), total length 71–76; length to notch 28–35; outer root well developed, length 38–45, inner root length 11–18, point length 9–11. Dorsal bar plate-shaped, total length 17–25, total width 11–23, median width ( Fig. 2C View Fig ). Ventral bar broadly V-shaped with notched edge, total length 14–20 (16, n =4), total width 7–10, median width 3–4 ( Fig. 2D View Fig ). Marginal hooks 7 pairs; hook length: pair I ( Fig. 2E View Fig ) 40–47; pair II ( Fig. 2F View Fig ) 30–34; pair III ( Fig. 2G View Fig ) 31–37; pair IV ( Fig. 2H View Fig ) 36–45; pair V ( Fig. 2I View Fig ) well developed, 60–64, pair VI ( Fig. 2J View Fig ) 37–43; pair VII ( Fig. 2K View Fig ) 39–48. Pair of needles ( Fig. 2L View Fig ) located near fifth hooks, length 10–13 (12, n =3).
Host. Silver carp Hypophthalmichthys molitrix ( Cypriniformes : Cyprinidae )
Site of infection. Gill rakers.
Molecular analysis. The partial 28S rDNA (731 bp) sequences from the two specimens were identical and submitted to the DNA Data Bank of Japan Centre (DDBJ) ( LC414156 View Materials ). Two species of Pseudodactylogyrus were used as the outgroup for the phylogenetic analysis, the tree agree with the part of analysis by Nitta and Nagasawa (2016), and Dactylogyrus skrjabini forms a sister group with D. hypophthalmichthys ( Fig. 3 View Fig ).
Remarks. This species was originally described from the gills of H. molitrix in the Amur River Basin, Far-East Russia ( Achmerow 1954). It was subsequently reported from the gills of the same host in the natural distribution range of the host: Lake Taihu, and Anhui, Hubei, Fujian, Beitun, Habahe, and Burqin provinces in China ( Long and Lee 1960; Lee 1963; Anonymous 1973a; Huang 1986; Zhao 2011). The dorsal anchor shape and well developed fifth marginal hook are characters to distinguish D. skrjabini from the other congeneric species, and the specimens examined in this study agree with the descriptions by Achmerow, (1954), Bykhovskaya-Pavlovskaya et al. (1962), Long (2000), and Gussev et al. (2010). The detailed internal anatomy of the species was firstly described herein and showed the common dactylogyrid form.
The present finding represents the first record of D. skrjabini from Japan. This monogenean is established along with H. molitrix in the European region of Russia ( Musselius 1969, 1973; Osmanov 1971), Turkmenistan ( Babayev 1964), Kazakhstan ( Gvozdev and Agapova 1977), Hungary ( Hoffman and Schubert 1984; Molnár 1984), Iraq ( Salih et al. 1988; Ali et al. 1989; Mhaisen et al. 2012; Al-Jawda and Asmar 2015), and the Aral Sea ( Urazbaev and Kurbanova 2006; Zonn et al. 2009).
Japanese name. The species is one of the biggest species in the genus, and the new Japanese name refers it: “dai” and “yubigata-mushi” mean large and the genus, respectively.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Monopisthocotylea |
|
Order |
|
|
Family |
|
|
Genus |
Dactylogyrus skrjabini Achmerow, 1954
| Nitta, Masato & Nagasawa, Kazuya 2020 |
Dactylogyrus scrjabini
| Anonymous 1973: 139 |
| Yukhimenko, S. S. 1972: 155 |
| Osmanov, S. O. 1971: 104 |
| Gussev, A. V. 1967: 56 |
| Babayev, B. 1964: 51 |
| Bykhovskaya-Pavlovskaya, I. E. & Gusev, A. V. & Dubinina, M. N. & Izyumova, N. A. & Smirnova, T. S. & Sokolovskaya, I. L. & Shtein, G. A. & Shul'man, S. S. & Epshtein, V. M. 1962: 254 |
Dactylogyrus skrjabini
| Mhaisen, F. T. & Al-Rubaie, A. L. 2016: 5 |
| Al-Jawda, J. M. & Asmar, K. R. 2015: 129 |
| Mhaisen, F. T. & Al-Niaeem, K. S. & Al-Zubaidy, A. B. 2012: 107 |
| Zhang, K. 2012: 123 |
| Al-Saadi A. A. J. & Hasan, H. R. 2010: 3 |
| Karabekova, D. U. 2008: 331 |
| Johnson, P. T. J. & Lunde, K. B. 2005: 132 |
| Grigorovich, I. A. & MacIsaac, H. J. & Shadrin, N. V. & Mills, E. L. 2002: 1208 |
| Xia, X. & Wang, W. & Lu C. 2000: 152 |
| Blanc, G. 1997: 497 |
| Gibson, D. I. & Timofeeva, T. A. & Gerasev, P. I. 1996: 29 |
| Ali, N. M. & Mhaisen, T. & Abul-Eis, E. S. & Kadim, L. S. 1989: 152 |
| Hoffman, G. L. & Schubert, G. 1984: 238 |
| Rohde, K. 1979: 655 |
| Gvozdev, E. V. & Agapova, A. I. 1977: 109 |
| Bauer, O. N. & Hoffman, G. L. 1976: 165 |
| Musselius, V. A. 1969: 237 |
| Akmetov, B. A. 1963: 462 |
| Lee, Y. 1963: 76 |
| Yamaguti, S. 1963: 30 |
| Long, S. & Lee, W. 1960: 218 |
| Bogdanova, E. A. 1957: 1391 |
| Achmerow, A. K. 1954: 168 |