Cybella bedosae, Judson, Mark L. I., 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4258.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:B1F45C56-A43A-4A8C-9190-2B861A37D33C |
|
DOI |
https://doi.org/10.5281/zenodo.6023805 |
|
persistent identifier |
https://treatment.plazi.org/id/E40C8CA5-237F-41B3-B7E1-D108F163933E |
|
taxon LSID |
lsid:zoobank.org:act:E40C8CA5-237F-41B3-B7E1-D108F163933E |
|
treatment provided by |
Plazi |
|
scientific name |
Cybella bedosae |
| status |
sp. nov. |
Cybella bedosae View in CoL n. sp.
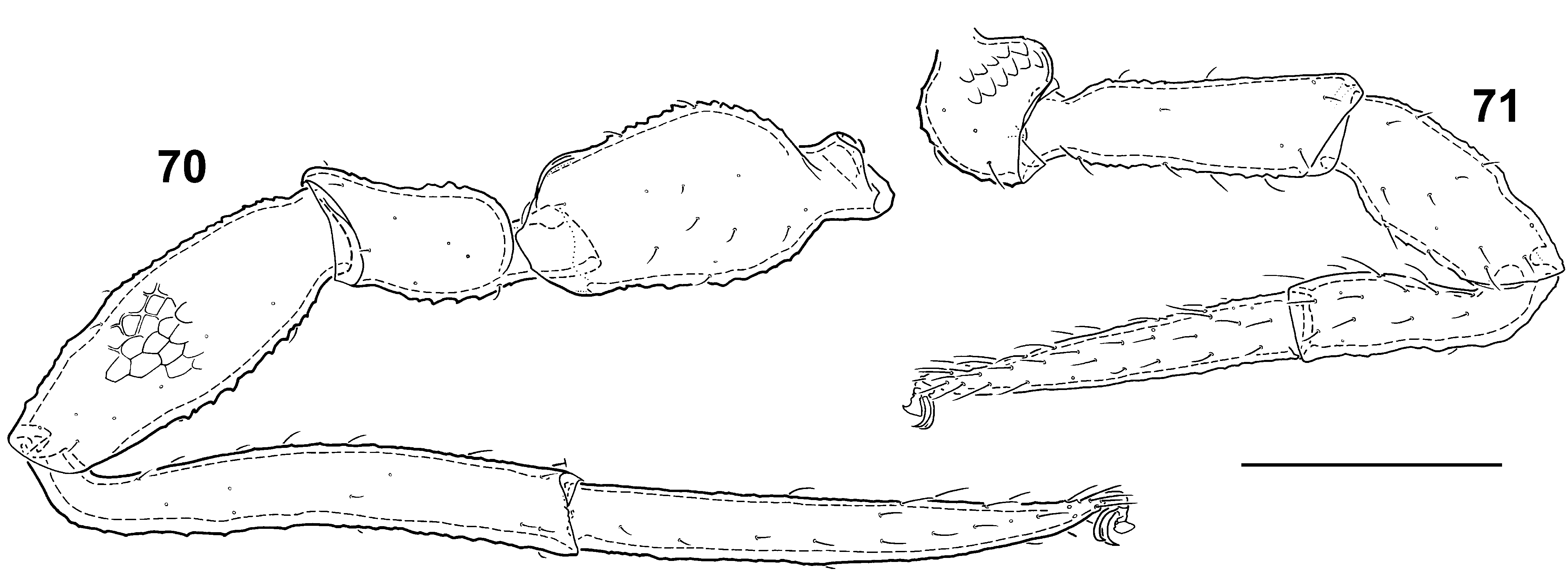
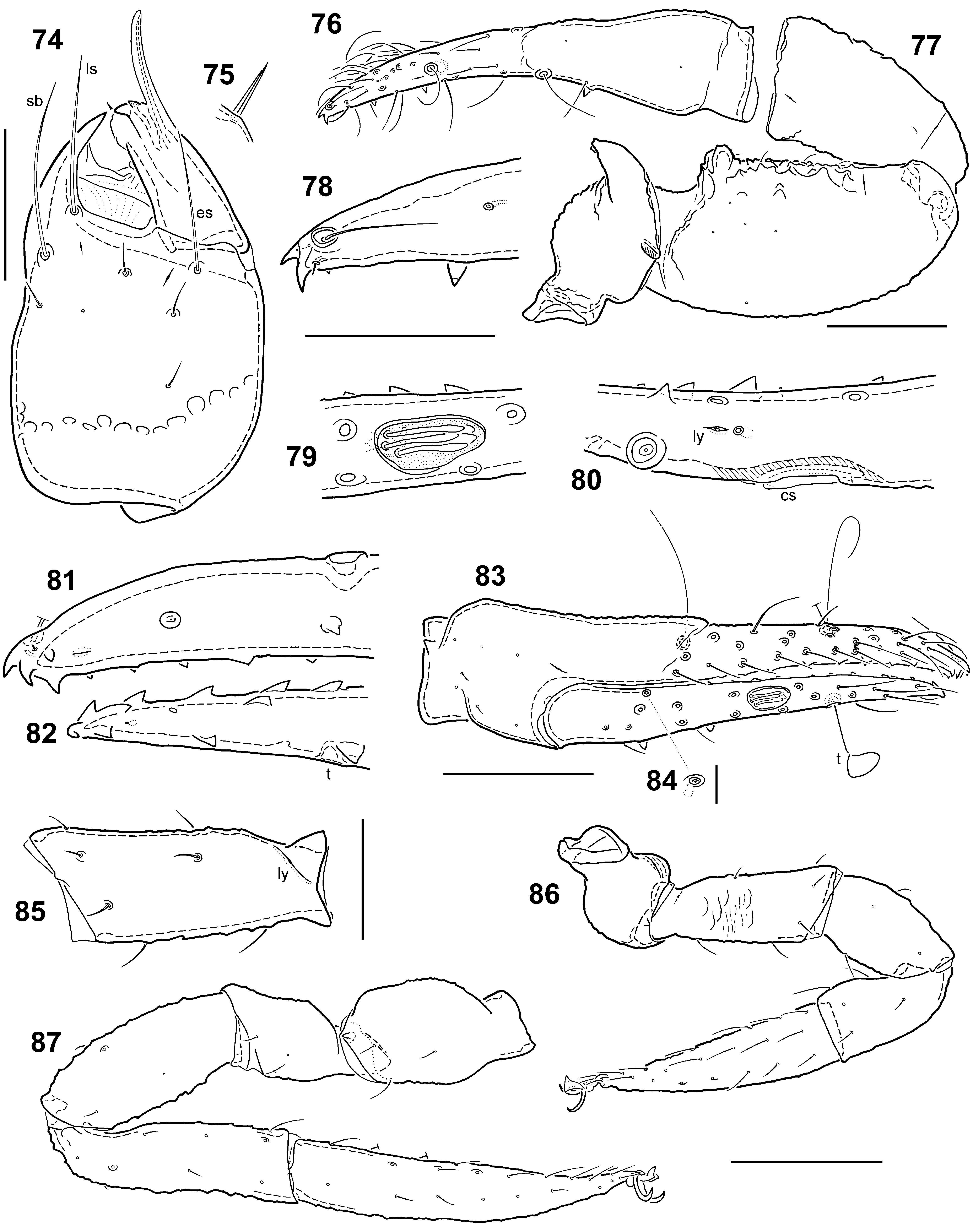
Figs 48 View FIGURES 48 ‒ 49 , 50‒87 View FIGURES 50 – 59 View FIGURES 60 – 69 View FIGURES 70 – 71 View FIGURES 72 – 73 View FIGURES 74 – 87
Diagnosis. Very similar to C. deharvengi n. sp., but larger (e.g. male palp femur length 0.573 versus 0.493–0.522 mm), with chela less robust (4.8 times longer than broad, versus 4.3–4.5), most segments of legs more attenuate (e.g. femur of leg IV 2.8 times longer than broad, versus 2.0–2.1), trichobothrium sb slightly proximad of pit (versus below pit), trichobothria eb and esb separated by more than one bothridial diameter (versus half a diameter), anterior genital sternite with 12 setae (versus 8), and lateral diverticulum of male atrial plate of longer.
Etymology. This species is named after Anne Bedos (MNHN), who helped collect the types and who has contributed much to our knowledge of the soil and cave faunas of SE Asia.
Type material. Holotype: ♂, Cambodia, Kampuchea, Kampot, Phnom Laang, Kien Krol , 1042ʹ14.4ʺN 10420ʹ41.1ʺE, 45 m a.s.l., Berlese extraction of soil from low passage (“boyau”) on left side of cave, 20 November 2005, leg. L. Deharveng & A. Bedos ( MNHN Ps116-01; collectors’ number KAM05-21) . Paratype protonymph (MNHN Ps116-02), collected with holotype.
DNA sequences. Partial sequences, obtained by non-destructive extraction from the palp of the paratype protonymph (voucher no. MNHN-JAD72), were deposited in GenBank for 18S RNA (accession no. JN018313.1) and 28S RNA (accession no. JN018410.1) by Arabi et al. (2012).
Description of male (limited to characters showing differences from C. deharvengi n. sp.). Tergal and sternal chaetotaxy not determined due to accumulation of exocuticle and debris, but where visible numbers of setae similar to those seen in C. deharvengi n. sp. Coxa I with 1 primary and 10–12 secondary spines around pit ( Figs 52, 55 View FIGURES 50 – 59 ). Coxal setae ( Fig. 52 View FIGURES 50 – 59 ): P 3 on manducatory process + 32 (total 35), I 8, II 9 (1 lateral), III 7 (1 lateral), 10 (1 lateral). Anterior genital sternite ( Fig. 52 View FIGURES 50 – 59 ) with 12 setae, posterior genital sternite ( Fig. 52 View FIGURES 50 – 59 ) with 14 setae (2+10+2). Male genitalia ( Fig. 58 View FIGURES 50 – 59 ) very similar to those of C. deharvengi n. sp., but comparison of dorsal apodeme, lateral rods and atrial plate hampered by differences in orientation in the specimens examined (this depending on the degree to which the genital plates are open or closed); lateral sacs longer and thinner than those of C. deharvengi n. sp.; lateral pockets of atrial plate slightly longer than those of C. deharvengi n. sp.; posterior plate with 1 pair of modified basal setae, which seems to be more rounded at the tip than those of C. deharvengi n. sp., and 2 small, simple setae on each side.
Chelicera ( Figs 60–64 View FIGURES 60 – 69 ) with 6‒7 small setae on palm, in addition to the 5 large setae. Galeal seta (gs) 0.85 from base, extending well beyond tip of spinneret (by nearly half length of hair). Spinneret ( Figs 61‒62 View FIGURES 60 – 69 ) slightly thicker than in C. deharvengi n. sp., but still tubular (i.e. not blade-shaped), length 16 µm. Serrula exterior with 19–20 blades, plus small distally-directed apical process. Serrula interior with 17 internal ducts; apically with a distallydirected process, followed by two rows of three, low, rounded mounds; basal blades forming a velum. Rallum with 1 (right chelicera) or 2 (left chelicera; Fig. 63 View FIGURES 60 – 69 ) blades, length 23 µm.
Palp as illustrated ( Figs 65–69 View FIGURES 60 – 69 ). Fixed chelal finger with 12 chemosensory setae in antiaxial row. Movable finger with 14–16 long (22–24 µm), spatulate, chemosensory setae in pit ( Fig. 67 View FIGURES 60 – 69 ). Trichobothria ( Figs 66, 68 View FIGURES 60 – 69 ) eb and esb separated by 1.2–1.8 bothridial diameters (absolute distance 17–22 µm); sb slightly proximad of posterior edge of pit. Fixed finger with 30 teeth: apodens plus 22 marginal, 6 paraxial and basal tuberculate tooth. Movable finger with 28 teeth: apodens plus 21 marginal, 7 paraxial and basal tuberculate tooth.
Legs as illustrated ( Figs 70‒71 View FIGURES 70 – 71 ).
Measurements (in mm, standard ratios in parentheses). Body (contracted) 1.82×1.25 (1.5). Carapace 0.659×0.47 (1.4). Chelicera 0.234×0.141 (1.7), palm 0.159 (1.1), movable finger 0.094. Palp femur 0.573×0.298 (1.8), patella 0.453×0.178 (2.5), chela 0.665×0.138 (4.8), palm 0.162 (1.2), movable finger 0.474 (2.9). Leg I femur 0.256×0.079 (3.3), patella 0.214×0.090 (2.4), tibia 0.212×0.062 (3.4), tarsus 0.304×0.051 (5.9). Leg IV trochanter 0.289×0.140 (2.1), femur 0.246×0.087 (2.8), patella 0.298×0.110 (2.7), tibia 0.426×0.063 (6.8), tarsus 0.418×0.052 (8.1).
Description of protonymph. Sclerotized parts pale straw-yellow, except tips of cheliceral fingers and teeth of chela, which are reddish-brown. Carapace ( Fig. 72 View FIGURES 72 – 73 ) distinctly broadened posteriorly; less elongate than in adult; anteromedian projections small, anterolateral corners of carapace more rounded than in adult; two pairs of lyrifissures; two pairs of eyes with reflective tapeta, anterior pair sessile, posterior pair weakly raised, both pairs less prominent than in adult. Tergite I ( Fig. 72 View FIGURES 72 – 73 ) with same general form as in adult, but presence or absence of separate lateral plate could not be determined due to weak sclerotization; tergites II–VIII divided (II and VIII weakly so); setae difficult to count, but most half-tergites with about 6 setae; denticulation present on tergites VIII– IX. Pleural membranes with two folds, as in adult, lyrifissures (orientated dorsoventrally) on dorsal fold; only 1 gland pore seen (anteriorly on dorsal fold), but others might be present; no setae observed; plates absent.
Palp coxa not as elongate as in adult, median maxillary lyrifissure present, with same form and position as that of adult; no lyrifissure distad of foramen. Coxa I with 1 primary and 9–10 secondary spines flanking pit ( Fig. 73 View FIGURES 72 – 73 ); no pores in pit. Coxae III reduced in size, reaching closer to midline than in adult, overlapped by coxae II. Palp coxa with 12 setae (including 3 on manducatory process), leg coxae each with 3 setae. Sternites IV–X divided, setae 0: 2: 2+2: 3+3: 3+3: 4+4: 4+4: 4+4: 4+4: 4 (2 flanking anal tubercle): 2 (slightly closer together than those of tergite XI); denticulate granulation on IX–XI.
Chelicera ( Figs 74‒75 View FIGURES 74 – 87 ) with distinction between smooth basal part and distal reticulate part of palm less well marked than in adult; three large (probably ls, sb and es) and four small setae on palm; movable finger without seta; rallum of 2 blades ( Fig. 75 View FIGURES 74 – 87 ), as in adult; spinneret ( Fig. 74 View FIGURES 74 – 87 ) simple, much longer (30 µm) than in adult, recurved apically. Serrula exterior with 10 blades plus apical process. Serrula interior with 10 ducts.
Palp ( Figs 76‒84 View FIGURES 74 – 87 ) similar to that of adult, trochanter with well developed horn, femur with moderate anterobasal projection. Chela with three trichobothria on fixed finger and one on movable finger. Trichobothrium x 1 inserted dorsally in shallow depression, hair straight and directed backwards, length 44 µm; trichobothrium t distinctly crooked ( Fig. 83 View FIGURES 74 – 87 ); hairs of other trichobothria (ist and it) lost or broken. Fixed finger with 21 teeth: apodens plus 14 marginal (irregular distally), 5 paraxial and basal tuberculate tooth. Movable finger with 21 teeth: apodens plus 15 marginal, 4 paraxial and basal tuberculate tooth. Tuberculate tooth of fixed finger bearing seta at base. Fixed finger with 3 lyrifissures: one on each side at base and 1 dorsal, just in front of trichobothrium it. Movable finger with 1 ventral lyrifissure, situated between t and pit; a single spot sensillum situated immediately behind lyrifissure ( Fig. 80 View FIGURES 74 – 87 ). Movable finger with 5 clavate chemosensory setae in a pit that is shallower than that of adult ( Figs 79‒80 View FIGURES 74 – 87 ). Proximal sensillum p 1 ( Fig. 84 View FIGURES 74 – 87 ) situated about a third from base of movable finger ( Fig. 83 View FIGURES 74 – 87 ); p 2 absent.
Legs ( Figs 85–87 View FIGURES 74 – 87 ) similar to those of adult (apart from usual differences), except that the trochanter of leg IV is relatively smaller and all femora lack the dorsal lyrifissure and its depression; femur of leg IV with large dorsoparaxial lyrifissure at base ( Fig. 85 View FIGURES 74 – 87 ) (as in adult).
Measurements (in mm, standard ratios in parentheses). Body 0.85×0.54 (1.6). Carapace 0.309×0.315 (0.98). Chelicera 0.129×0.082 (1.6), palm 0.092 (1.1), movable finger 0.062 (0.7×palm), spinneret 0.030 (0.5×movable finger). Palp femur 0.249×0.168 (1.5), patella 0.197×0.101 (1.9), chela 0.364×0.088 (4.1), palm 0.090 (1.0), movable finger 0.278 (3.1). Leg I trochanter 0.095×0.064 (1.5), femur 0.118×0.045 (2.6), patella 0.093×0.052 (1.8), tibia 0.093×0.048 (2.0), tarsus 0.173×0.041 (4.2). Leg IV trochanter 0.128×0.069 (1.8), femur 0.095×0.051 (1.9), patella 0.136×0.057 (2.4), tibia 0.166×0.045 (3.7), tarsus 0.230×0.042 (4.5).
Remarks. An attempt by Anne Bedos and the author to collect more material of C. bedosae n. sp. at the type locality in June 2008 was thwarted by dense brush outside the cave, which had grown since the previous visit in 2005, due to the cessation of grazing activity at the site. Berlese extractions of litter and soil samples taken just outside the cave in 2005 and nearby in 2008 did not produce any feaellids.
Although C. bedosae n. sp. and C. deharvengi n. sp. are very similar and known from only a few specimens, they appear to be isolated geographically, occurring in disjunct limestone hills about 60 km apart. Feaellidae appear to have very poor powers of dispersal and show high levels of endemism, so it seems reasonable to infer that two closely related species are involved, rather than a single variable or clinal species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |