Ctenobrycon oliverai, Benine, Ricardo C., Lopes, Guilherme A. M. & Ron, Ernesto, 2010
|
publication ID |
https://doi.org/ 10.5281/zenodo.199703 |
|
DOI |
https://doi.org/10.5281/zenodo.5668677 |
|
persistent identifier |
https://treatment.plazi.org/id/72328796-E90A-FF9F-FF78-932D7D910339 |
|
treatment provided by |
Plazi |
|
scientific name |
Ctenobrycon oliverai |
| status |
sp. nov. |
Ctenobrycon oliverai View in CoL , new species
Table 1, Figs. 1–2 View FIGURE 1 View FIGURE 2
Holotype. MZUSP 50130, 54.4 mm SL, río Apure, West of Ciudad de Apure, State of Apure, Venezuela. 28 January 1982. O. Costillo et al.
Paratypes. MZUSP 27979, 15, 38.7 – 55.8 mm SL, collected with the holotype. LBP 3061, 11, 43.8 – 54.6, 1 C&S, 50.3 mm SL, Rio Orinoco, Ciudad de Caicara del Orinoco, 07°38’11.6” N, 66°19’04.2”. State of Bolívar, Venezuela. 0 3 October 2005. A. Granado & C. Oliveira.
Diagnosis. Ctenobrycon oliverai is distinguished from all congeners and Psellogrammus kennedyi by the number of scale rows between dorsal-fin origin and lateral line (14 – 15 vs. 11 – 13 in C. spilurus ; 11 – 12 in C. alleni and P. kennedyi ). Ctenobrycon oliverai is further distinguished from C. alleni by the number of humeral blotches (one vs. two, respectively). The following characters may be usefull in distinguishing C. oliverai from congeners: greatest body depth (48.0–58.3% of SL vs. 41.3–53.0 in C. spilurus ; 41.1–51.0% of SL in C. alleni ; 42.4–50.5% of SL in P. kennedyi ).
Description. Morphometric data for Ctenobrycon oliverai are summarized in Table 1. Deep bodied. Greatest body depth at dorsal-fin origin. Dorsal profile of head concave. Dorsal profile of body strongly convex from tip of supraoccipital spine to dorsal-fin origin, dorsal-fin base posteroventrally slanted, straight or slightly convex from posterior terminus of dorsal-fin base to end of adipose fin, and concave along caudal peduncle. Ventral body profile convex from tip of lower jaw to anal-fin origin, anal-fin base posterodorsally slanted, concave along caudal peduncle. Prepelvic region transversally flattened, more so proximal to pelvic-fin insertion. Postpelvic region transversally flattened proximal to pelvic-fin insertion becoming somewhat obtuse toward anal-fin origin.
holotype N paratypes
Limits mean Standard length (mm) 55.4 28 39.4–59.9 50.1 Percentage of head length
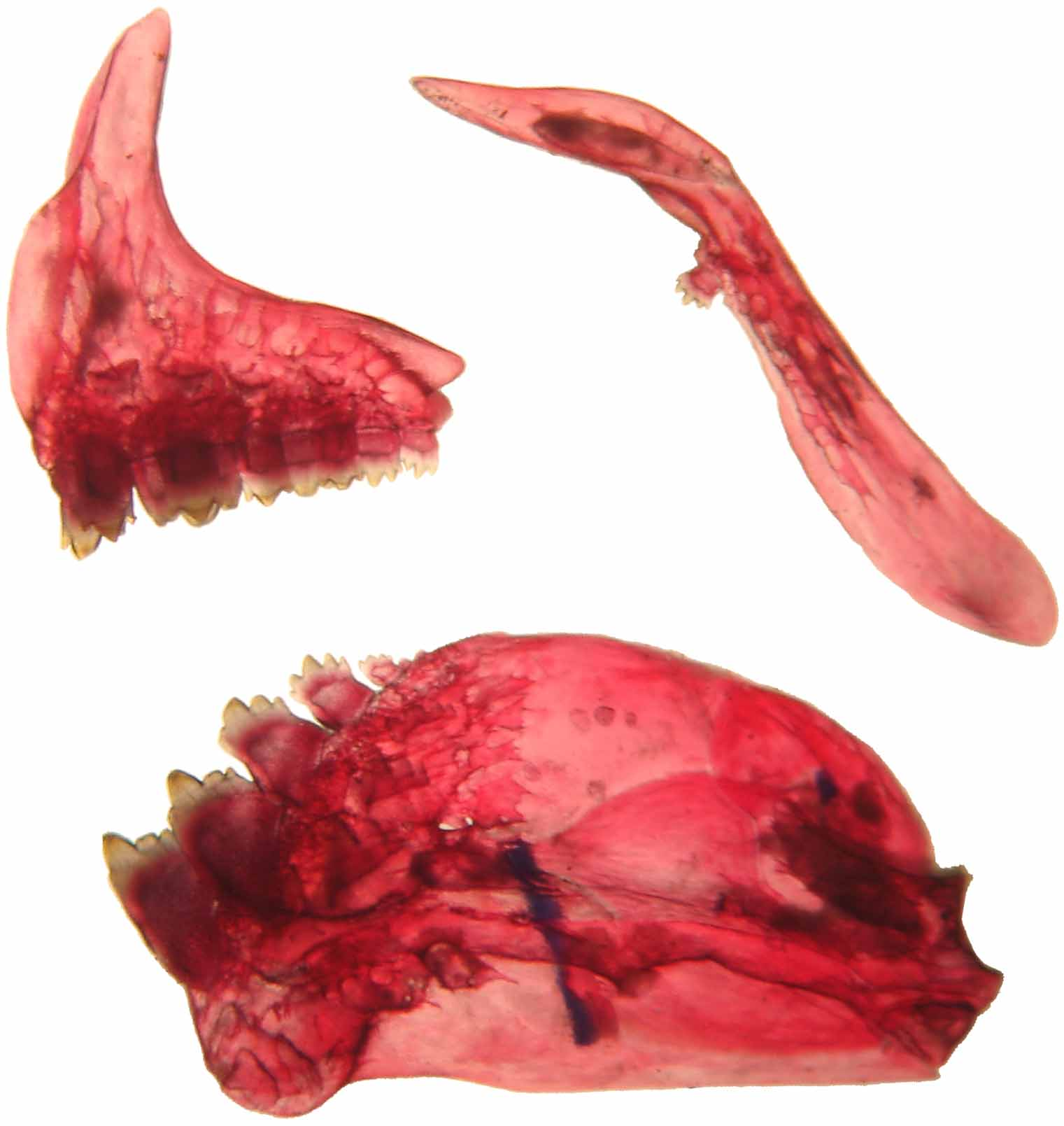
Snout length 25.2 28 21.5–26.9 24.5 Upper jaw length 35.6 28 34.6–39.8 36.3 Horizontal orbital diameter 38.6 28 37.7–43.1 40.6 Least interorbital width 41.6 28 37.4–42.8 40.2 Mouth terminal. Maxillary not surpassing vertical through anterior margin of orbit. Premaxillary teeth in to rows; outer row with 3 (2), 4* (25), 5 (1) tricuspid teeth, midcentral cusps longer than others; inner tooth row with 5* (27), 6 (1) teeth with 3 to 6 cusps, midcentral cusps longer than others. Maxillary with 1* (27) or 2 (1) pentacuspidate teeth. Dentary with 5 teeth with 3 to 5 cusps usually midcentral cusps longer than others, followed by 1 to 3 small teeth, with 1 to 3 cusps ( Fig. 2 View FIGURE 2 ).
Nostrils closer to anterior orbital margins than to each other. Supraoccipital process elongate, its tip surpasses the vertical through origin of pectoral fin.
Dorsal-fin rays ii,9. Pectoral-fin rays i,11 (3), i,12* (17), i,13 (8). Tip of pectoral fin exceed anterior half of length of adpressed pelvic fin. Adipose fin well developed. Pelvic-fin rays i,7, when adpressed, its tip extends up to first branched ray of anal fin. Anal-fin rays iv, 39 (2), 40 (1), 41 (1), 42 (2), 43 (8), 44 (7), 45 (3*), 46 (1). Principal caudal-fin rays i,17,i. Caudal fin forked.
Spinoid scales. Lateral line complete, 51 (1), 52 (2*), 53 (3), 54 (2), 55 (2), 56 (5), 58 (1). Scale rows between dorsal-fin origin and lateral line 14 (17*), 15 (7), scale rows between lateral line and pelvic-fin origin 11 (11*), 12 (13). Circumpeduncular scale rows 18 (1*), 19(5), 20 (2), 21 (1). Scale sheath along anal-fin base in a single series, extending posteriorly between 33–42* branched anal-fin ray.
First gill arch with 14* (12), 15 (3), 16 (1) on upper limb and 8* (11), 9 (5) on lower limb. Total vertebrae 32, supraneurals 4.
Sexual dimorphism. No secondary sexually dimorphic feature, such as bony hooks on anal and pelvic fins, were observed in Ctenobrycon oliverai .
Color in alcohol. Overall coloration yellowish. Mid-dorsal line darker. Scattered small dark chromatophores on dorsal surface of head from upper lip to tip of supraoccipital spine. Infraorbitals, preopercle, and opercle retaining guanine. Lower lip well delimited by dark chromatophores. Small dark chormatophores delineating inferior margin of eyes. Ventral portion of head with very few dark chromatophores, more concentrated on branchiostegal rays.
Wedge-shaped humeral mark extending horizontally from fourth to sixth scale posterior to opercle and vertically from the first to seventh scale series above lateral line. A two scales-deep silver midlateral stripe extending on portion of body beginning at vertical through the first branched dorsal-fin ray, with stripe narrowing posteriorly up to a vertical oval-shaped dark spot on terminus of caudal peduncle. Limits of the erector/depressor muscles of the anal fin outlined by dark chromatophores.
Dorsal fin with scattered small dark chromatophores uniformly distributed in its interradial membrane; second unbranched dorsal-fin ray dark pigmented with chromatophores distributed along its whole extension; first branched dorsal-fin ray with dark pigments restricted to its distal half; further dorsal-fin rays hyaline. Anal fin with few dark chromatophores uniformly distributed in its interradial membrane in most specimens; anal-fin hyaline or with very few sparsely distributed dark chromatophores in few specimens. Caudal fin with small chromatophores sparsely distributed in its interradial membrane. Adipose fin hyaline or with small chromatophores, more concentrated in its distal half. Pectoral fins with small chromatophores homogeneously distributed along the unbranched ray; small chromatophores in the distal half of the first six branched rays; interradial membrane hyaline. Pelvic fin hyaline.
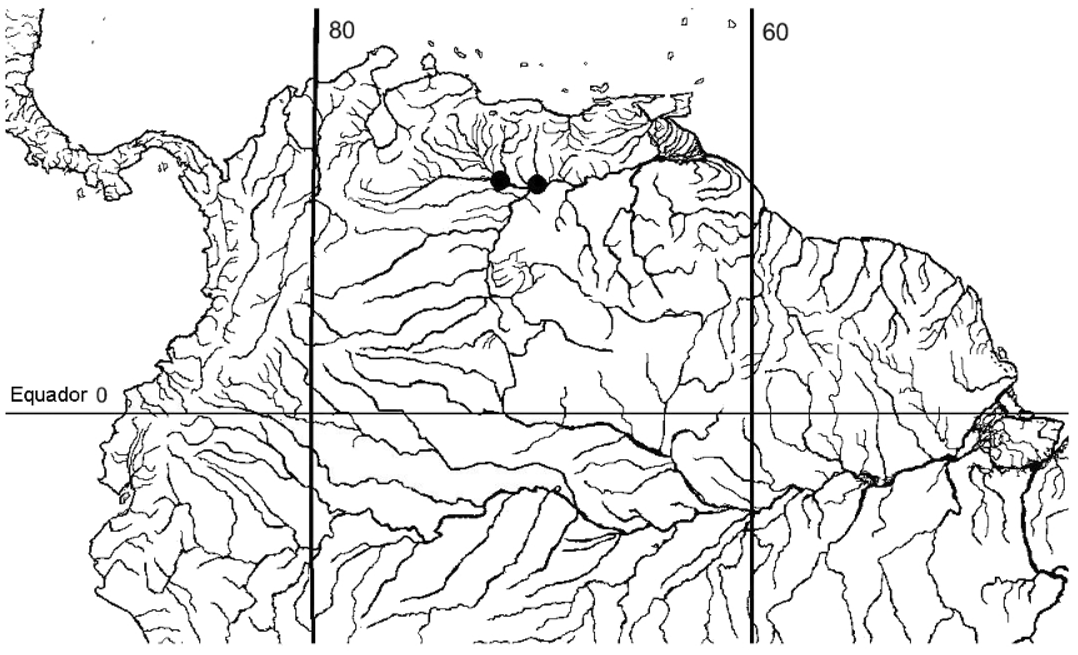
Distribution. Known from the río Orinoco and río Apure, río Orinoco basin, Venezuela ( Fig. 3 View FIGURE 3 ).
Etymology. The specific name is in honor of Claudio de Oliveira, the collector of the new species and a great contributor to our knowledge of Neotropical Ichthyology.
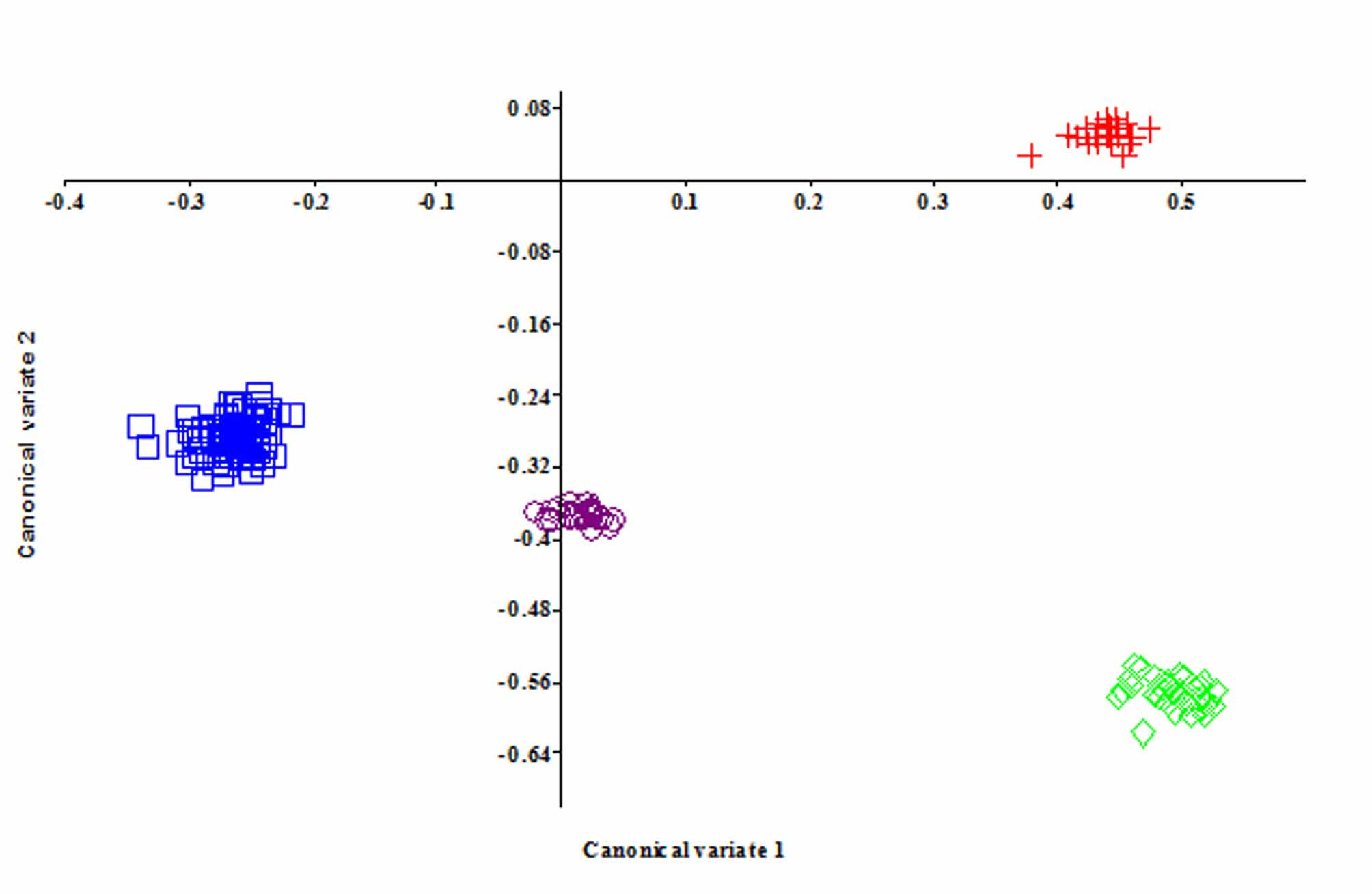
Multivariate analysis. The results of the size-free canonical variate analysis revealed that Ctenobrycon oliverai can be discriminated from C. alleni , C. spilurus – hauxwellianus and from P. kennedyi in the first and second canonical variate axis that explains 68.23% and 23.22% of the total variance of the data, respectively ( Fig. 4 View FIGURE 4 ; Table 2 View TABLE 2 ). Ctenobrycon oliverai and C. alleni have greater anal fin-length, least interorbital width, and eye to origin of dorsal-fin distance (higher positive values of CV1, p<0.05) than that found in C. spilurus-hauxwellianus and Psellogrammus kennedyi . These last species in turn present higher snout to dorsal-fin origin distance than C. oliverai and C. aleni (higher negative value of CV1, p<0.05). In addition, C. oliverai could be discriminated from the other three species on the second canonical axis, based on higher body depth (higher positive values of CV2, p<0.05) whereas, C. spilurus-hauxwellianus, C. alleni and P. kennedyi presented greater head depth and head length than C. oliverai (higher negative value of CV2, p<0.05).
Discussion. Eigenmann (1927:330–336), in the first revisionary study after the original description of the genus, recognized Ctenobrycon hauxwellianus (Cope) , from Amazon basin; C. spilurus (Valenciennes) , from Venezuela and Suriname; and C. multiradiatus (Steindachner) , from Amazon basin and possibly Paraguay basin. This author diagnosed C. hauxwellianus from C. spilurus uniquely by the average of the body depth (2.0 times in SL versus 2.5 times in SL, respectively). Eigenmann, however, was not sure about the validity of C. multiradiatus , since he considered that the diagnostic characters used by Steindachner could be purely individual variations and also pointed out that the forms from Paraguay were most likely Astyanax alleni Eigenmann & MacAtee , or A. pelegrini Eigenmann or even Psellogrammus kennedyi Eigenmann. Géry (1977) , reallocated Astyanax alleni in Ctenobrycon but, due to the great overlap in the characters, named the subspecies C. spilurus spilurus , C. spilurus hauxwellianus , and C. spilurus alleni . Besides, this author also cited C. multiradiatus and C. correntinus as valid, but argued that these were probably synonyms of C. hauxwellianus and Astyanax pelegrini , respectively. Notwithstanding the syntypes of C. spilurus and C. hauxwellianus are inadequately preserved, which further hinders the process of delineating these species, we herein recognized C. hauxwellianus ( Cope, 1870) as a junior synonym of C. spilurus (Vallenciennes) , based on the examination of the comparative lots of Ctenobrycon , which did reveal a substantial overlap for all morphometric and meristic characters, not supporting the diagnostic characters presented by Eigenmann (1927) for C. spilurus and C. hauxwellianus . Ctenobrycon alleni , however, could be easily distinguished from congeners by presenting two humeral blotches versus a single one, a condition already described by Britski, 2007.
Taking into account this great overlap in characters, a given count that falls totally out of the known range of Ctenobrycon is strong evidence that it is related to a different species. From the 235 examined comparative specimens, all of them presented up to 13 scale rows above the lateral line (vs. 14 –15 in C. oliverai ). Moreover, no comparative material from río Orinoco drainage has more than 12 scale rows above lateral line, which enhance our decision of considering this species as new rather than a population which would only widen the variation range observed for Ctenobrycon spilurus . The results of the multivariate analysis corroborate our hypothesis showing statistically significant evidences which discriminate Ctenobrycon oliverai from C. spilurus and Psellogrammus kennedyi .
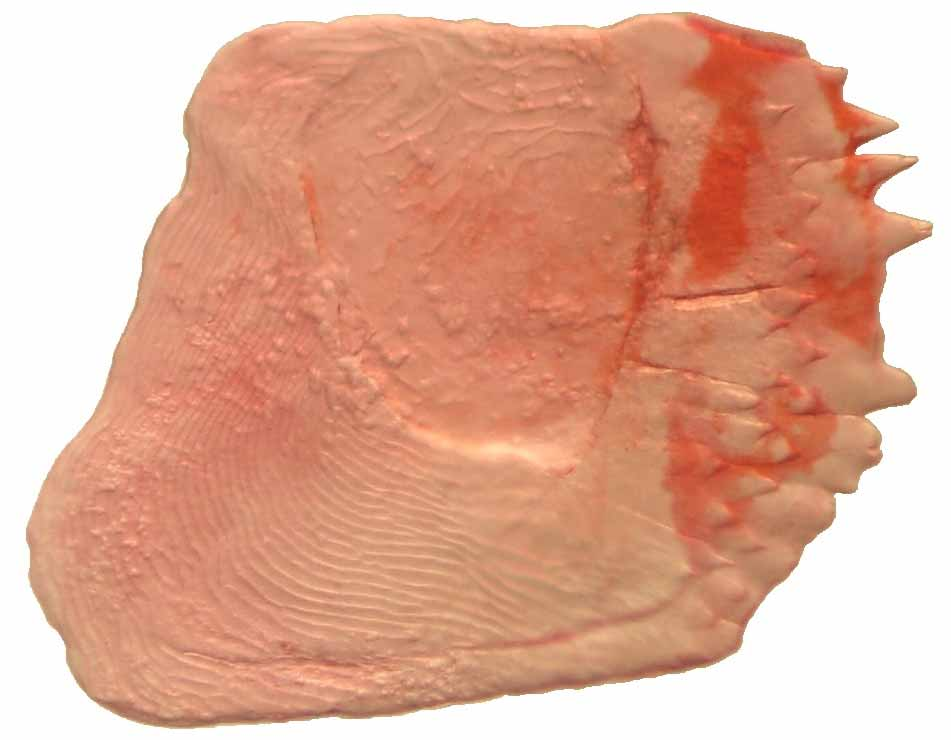
Lima et al. (2003) listed Tetragonopterus correntinus Holmberg , T. gibbicervix, Pelegrin , T. multiradiatus Steindachner and Astyanax pelegrini Eigenmann as species inquirendae in Ctenobrycon . Nonetheless, Mirande et al. (2006) considered both Tetragonopterus correntinus and Astyanax pellegrini , species of Astyanax rather than Ctenobrycon , since these do not display the spinoid scales, which promptly separate these taxa from our new species (that presents spinoid scales). According to Mirande’s (2010) examinations, the scales of the belly of Psellogrammus kennedyi have simple flattened serrations restricted to the margin of the scales similar to that of crenate scales in the classification of Roberts (1993). However, our examinations indicated that the form of the scales of the belly in both Ctenobrycon and Psellogrammus species are, in fact, spinoid, with acute projections not restricted to the margin of the scales ( Fig. 5 View FIGURE 5 ).
Examination of images of three syntypes of Tetragonopterus gibbicervix , made it clear that this species presents 12 scale rows above lateral line, which distinguishes it from our new species (that presents 14–15 scale rows above lateral line).
Géry (2006) affirmed that Tetragonopterus multiradiatus Steindachner is a junior synonym of C. hauxwellianus , but did not expose the reasons for considering so, nor de he mention the paper where this new combination was proposed, if it exists at all. Considering that the syntypes of Tetragonopterus multiradiatus are possibly lost (see Lima et al., 2003), not much information can be retrieved but from Steindachner’s original description and putative topotypes. The eight examined specimens from the type locality (MZUSP 27765, município de Tefé, AM, Brazil) presented one tooth in the maxillary bone, 45 to 49 scales in the lateral line, and 11 to 12 scale rows above lateral line and 9 to 10 scale rows below lateral line, perfectly fitting with C. hauxwellianus (= C. spilurus ), according to the key presented by Eigenmann. In fact, none of the herein examined morphotypes of Ctenobrycon presented toothless maxillaries and 41–42 lateral line scales as described for T. multiradiatus by Steindachner (1876) and, thus, there are not enough elements to assure that C. multiradiatus is a junior synonym of C. spilurus or even if it is a species of Ctenobrycon , if we consider that no mention was made on the presence of spinoid scales in C. multiradiatus , be it in its original description or elsewhere.
Comparative material. Ctenobrycon alleni : LIRP 3786 (n=1), Brasil, Mato Grosso do Sul, Porto Manga, rio Paraguai; MZUSP 54023 (34) (1 C&S), Paraguai, Concepcion, Puerto Itacua, rio Paraguai; Ctenobrycon spilurus : Brazil: MZUSP 5156 (28), Roraima, Surumu, rio Surumu; MZUSP 5601 (22), Pará, Oriximiná, rio Trombetas, Lago Parú; Amazonas: LIRP 4999 (21) (3 C&S), Janauari, Lago Terra Preta; LIRP 4965 (10), Janauaca, Lago Castanho; LIRP 4985 (2), Camaleão, Ilha de Marchantaria, rio Amazonas; MZUSP 27765 (8), Tefé, baixo rio Japurá; MZUSP 54495 (8), Equador, Napo, rio Yasuní; Brazil, Acre, Cruzeiro do Sul, rio Moa: LBP 4047 (4), (1 C&S) LBP 4151 (2), rio Japiim: LBP 4095 (20); Peru, Ucayali, Província de Coronel Portilho, rio Ucayali: MZUSP 25996 (1); MZUSP 26242 (8); Suriname, Paramaribo: ANSP 137053 (3); Venezuela, Guárico: MCP 15138 (5), Camaguán, río Portuguesa; MZUSP 74698 (3), San Fernando, río Guárico; Bolívar, Caicara Del Orinoco: LBP 2232 (11), (2 C&S), río Orinoco; LBP 2222 (2), Laguna de Castilleros; Psellogrammus kennedyi : LBP 3220 (24) (3 C&S), Brasil, Mato Grosso, Nobres, rio Cuiabazinho, Lagoa Marginal; Tetragonopterus alleni : FMNH 52634, paratype, Brasil, Mato Grosso, Corumbá. Tetragonopterus gibbicervix : NMW 57516, syntype, Brazil, Amazonas, Teffé (photo), MNHN 1909-182, paratype (photo); MNHN 1909-320/321, 2 paratypes (photos); Tetragonopterus hauxwellianus : ANSP 8138-8142, 5 paratypes, Pebas, Peru. Tetragonopterus spilurus : MNHN 5341, syntype (photo).
TABLE 2. Loadings of variables in the first and second size-free Canonical Varietes of Ctenobrycon oliverai, C. alleni, C. spilurus, C. hauxwellianus and Psellogrammus kennedyi.
| CV1 | Prob. | CV2 | Prob. | |
|---|---|---|---|---|
| Body depth Snout to dorsal-fin origin | 0.3625 -0.0934 | 0.6293ns 0.0115* | 0.3242 0.0718 | 0.0435* 0.0181* |
| Snout to anal-fin origin Pelvic-fin length | 0.1383 0.1751 | 0.5631ns 0.0749ns | -0.5182 0.1891 | 0.0000* 0.8191ns |
| Anal-fin length | 0.7619 | 0.0000* | -0.0966 | 0.0620ns |
| Anal-fin base Eye to dorsal-fin origin | 0.1178 0.1283 | 0.8807ns 0.0135* | 0.5496 -0.2729 | 0.0978ns 0.0001* |
| Dorsal-fin origin to caudal-fin origin Head depth | 0.1385 0.2928 | 0.3194ns 0.4602ns | 0.0673 -0.3413 | 0.1675ns 0.0000* |
| Head length | 0.1448 | 0.9687ns | -0.0230 | 0.0026* |
| Least interobital width Upper jaw length | 0.2652 0.0523 | 0.0043* 0.2473ns | 0.2115 0.1822 | 0.3815ns 0.1082ns |
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
| LIRP |
Laboratorio de Ictiologia, Faculdade de Filosofia |
| ANSP |
Academy of Natural Sciences of Philadelphia |
| MCP |
Pontificia Universidade Catolica do Rio Grande do Sul |
| FMNH |
Field Museum of Natural History |
| NMW |
Naturhistorisches Museum, Wien |
| MNHN |
Museum National d'Histoire Naturelle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |