Colposcenia dioscoridis, Malenovský & Burckhardt, 2014
|
publication ID |
https://doi.org/ 10.5281/zenodo.5314350 |
|
publication LSID |
lsid:zoobank.org:pub:44343D04-2985-45F4-BA26-4F5C3B481BDAD |
|
DOI |
https://doi.org/10.5281/zenodo.6344465 |
|
persistent identifier |
https://treatment.plazi.org/id/03C787CE-FFB2-8E37-FE6E-C97990389642 |
|
treatment provided by |
Marcus |
|
scientific name |
Colposcenia dioscoridis |
| status |
sp. nov. |
Colposcenia dioscoridis View in CoL sp. nov.
( Figs 1–3 View Figs 1–5 , 6–17 View Figs 6–9 View Figs 10–17 )
Type locality. Yemen, north-eastern Socotra, Haalla coastal area, thickets of Tamarix nilotica in Arher spring environs, 12°33′00″N 54°27′36″E, 5–15 m a.s.l. ( Figs 4–5 View Figs 1–5 ).
Type material. HOLOTYPE: ♂ ( MMBC, dry-mounted), ‘ YEMEN, SOCOTRA Island / Halla area , Arher / 12°33.0′N, 54°27.6′E, 5 m / freshwater spring in sand dunes / 9.–10.vi.2012 // on Tamarix nilotica / I. Malenovský leg.’. GoogleMaps PARATYPES: 14 ♂♂ 7 ♀♀, same data as the holotype; GoogleMaps 2 ♂♂ 3 ♀♀, southern Socotra , northern edge of Noged plain , Deiqab cave entrance , 12°23′03″N 54°00′56″E, 115 m, 12.vi.2012, on Tamarix nilotica , I. Malenovský leg.; GoogleMaps 2 ♂♂ 3 ♀♀, southern Socotra , Noged plain, Abataro, 12°22′06″N 54°03′24″E, 20 m, 12.–13.vi.2012, sand dunes , on Tamarix nilotica , I. Malenovský leg. ( MMBC, NHMB, NMPC; dry- and slide-mounted and preserved in alcohol). GoogleMaps
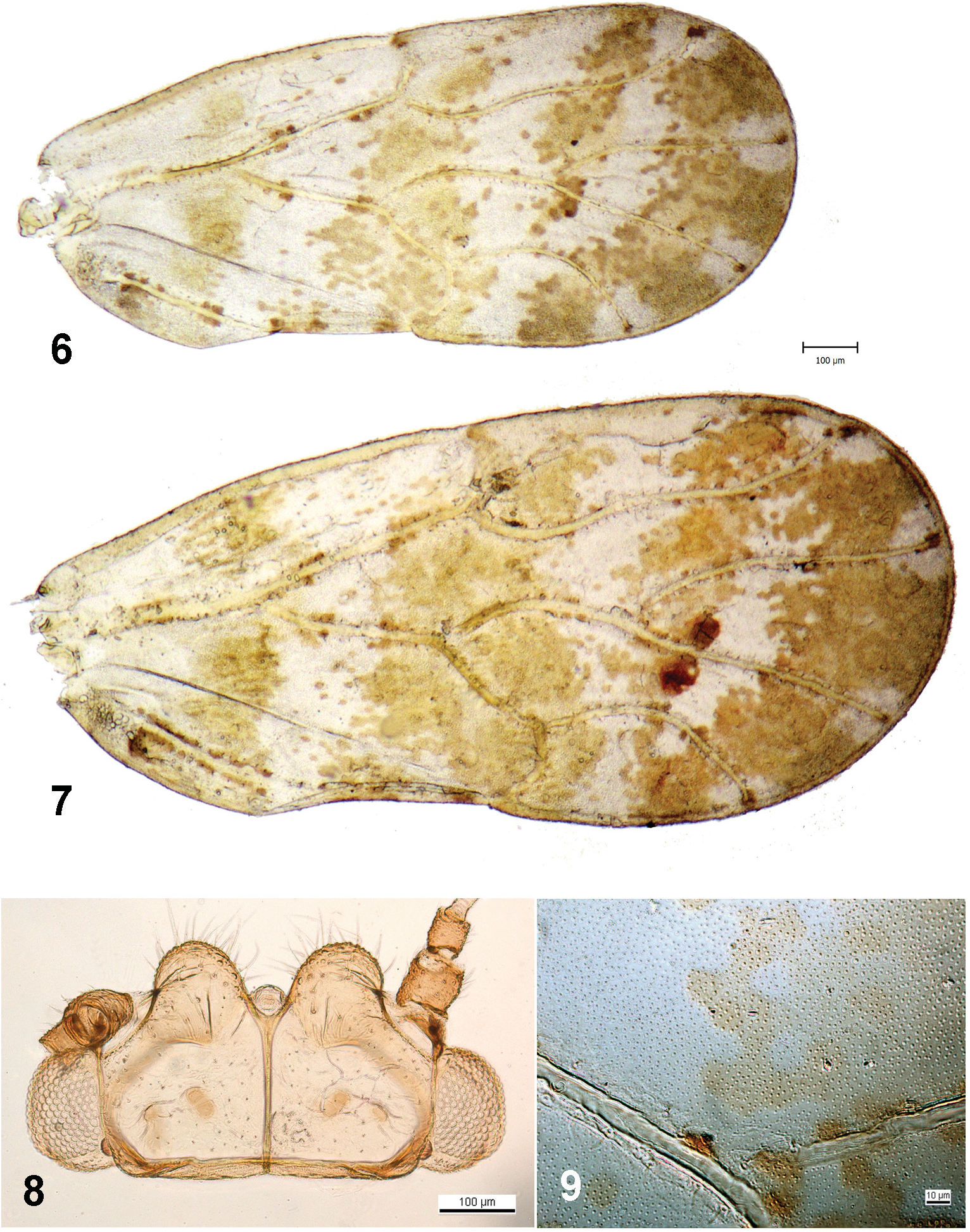
Description. Adult. Coloration ( Figs 1–3 View Figs 1–5 ). Body almost uniformly light green when alive, dirty yellow in specimens which are dried or preserved in alcohol. Foveae on vertex and pronotum and anterior parts of mesopraescutum and mesoscutum (in some specimens) faintly light brown. Eyes grey, ocelli red. Antenna dirty yellow with segments 1, 2 ventrally, segments 3–8 apically and segments 9, 10 entirely dark brown. Apical segment of rostrum dark brown to black. Legs uniformly dirty yellow, only apices of both tarsal segments infuscate dark brown and apical spurs on metatibia and metabasitarsus black. Forewing membrane opaque, milky white, with pattern consisting of many small light to dark brown spots, fused into four irregular transverse bands near base, medially, subapically and apically, the apical band leaving clear round patches around apices of veins R S, M 1+2, M 3+4 and Cu 1a; two small, more or less round, darker brown spots on membrane in cells m 1 and m 2 on either side of vein M 3+4 slightly distal to M fork, distinct and well-delimited particularly in females, which generally have a more contrasting wing pattern than males ( Figs 6, 7 View Figs 6–9 ); veins dirty yellow, apices of all veins bearing a small dark brown spot, several small dark spots also on the anal vein A
1. Hindwing hyaline, vein C+Sc brownish.
Structure. Integument with fine microsculpture, matt, covered with short inconspicuous light pubescence; anterior margin of vertex and genae with numerous long setae ( Fig. 8 View Figs 6–9 ). Head only slightly inclined from longitudinal body axis. Vertex flat, with large, flattened, apically broadly rounded anterior lobes (slightly longer than half of vertex length along midline), one small deep fovea on each side of median suture; lateral ocelli lying in plane of vertex, frontal ocellus clearly visible in dorsal view ( Fig. 8 View Figs 6–9 ). Genae quite flat, anteriorly and laterally rounded. Eyes subglobular. Antenna ( Fig. 14 View Figs 10–17 ) about 1.1–1.2 as long as head width, with 10 segments; segments 3–9 slightly widening to apex; segment 3 longest, segment 4 slightly shorter than 3 but longer than each of segments 5 and 6, segments 7 and 8 each shorter than 5 and 6; single elongate oval rhinarium bordered with wreath of small cuticular spines subapically on each of segments 4–9; one long and one shorter simple seta subapically on each of segments 3–8, segment 3 also with long simple seta medially; segment 10 with terminal setae subequal, longer seta about twice longer than segment 10 ( Fig. 15 View Figs 10–17 ). Clypeus nearly flat, rostrum short, both clypeus and rostrum lacking conspicuous setae. Metacoxa with relatively long, apically blunt meracanthus. Metatibia distinctly widening towards apex, rugged basally but lacking genual spine, with 5 dark sclerotised spurs apically – three grouped together on inner side, each of the remaining two spurs separated on outer side of metatibia. Metabasitarsus laterally bearing two dark sclerotised apical spurs. Forewing ( Figs 6, 7 View Figs 6–9 ) elongate oval, slightly widening apically, broadest in apical quarter, broadly and almost symetrically rounded apically, with apex lying in cell m 1; pterostigma well-developed, long, extending for approximately three quarters of length of cell r 1; vein R S sinuate medially, apex curved obliquely anteriad, ending on outer anterior wing margin; vein Cu 1b shorter than Cu; forewing membrane densely covered with small round surface spinules which are irregularly arranged ca. 3.0–3.5 μm apart in middle of cells and extend up to veins; forewing veins clothed with many short setae ( Fig. 9 View Figs 6–9 ). Hindwing costal margin with 1 + 3 setae basally, 4 setae medially and 1 seta apically. Male subgenital plate with nearly straight dorsal margin and few sparse short setae postero-ventrally ( Fig. 10 View Figs 10–17 ). Male proctiger robust with posterior lobes distinctly shorter than subgenital plate, slightly curved medio-dorsad and narrowing to a subacute apex; proctiger on outer as well as inner side beset only with fine setae, spine-like setae, present subapically on posterior proctiger margin in many other Colposcenia spp. , are missing ( Fig. 10 View Figs 10–17 ). Paramere, in lateral view, short, nearly parallel-sided, with apical part strongly produced posteriorly; inner side with several long, fine setae and a sclerotised, finger-shaped subapical process anteriorly ( Fig. 11 View Figs 10–17 ); in dorsal view, the posterior lobe is broadly rounded and bearing relatively long setae, and the finger-shaped anterior process oriented medio-posteriad ( Fig. 12 View Figs 10–17 ). Distal segment of aedeagus with a membraneous lobe on dorsal side of apical dilatation; ductus ejaculatorius short, bent dorsally ( Fig. 13 View Figs 10–17 ). Female terminalia with proctiger, in lateral view, with dorsal margin slightly concave, apex subacute; circumanal pore ring elliptic with two contiguous rows of pores; subgenital plate, in lateral view, regularly convex ventrally, with apex pointed ( Fig. 16 View Figs 10–17 ); dorsal and ventral valvulae slightly curved ventrally, smooth, lacking any teeth ( Fig. 17 View Figs 10–17 ).
Measurements (in mm). Males (n = 2): HW 0.59, AL 0.65–0.71, WL 1.35, WW 0.58, TL 0.34–0.37, MPL 0.16–0.17, PL 0.11, AEL 0.14. Ratios: AL/HW 1.20, WL/HW 2.29, WL/ WW 2.33, TL/HW 0.63, MPL/HW 0.29. Females (n = 2): HW 0.61–0.63, AL 0.66–0.67, WL 1.61–1.68, WW 0.68, TL 0.39, FPL 0.51–0.52, SL 0.37–0.41. Ratios: AL/HW 1.06–1.08, WL/HW 2.63–2.67, WL/WW 2.37–2.47, TL/HW 0.62–0.64, FPL/HW 0.83–0.84, FPL/SL 1.24–1.41.
Fifth instar immature unknown.
Differential diagnosis. Colposcenia dioscoridis sp. nov. belongs to Colposcenia vicina group characterised by BURCKHARDT (1988) as having short posterior processes of the male proctiger not extending beyond the subgenital plate, slightly curved medio-dorsad and gradually narrowing from base to apex (type V of LOGINOVA 1974); paramere shorter than proctiger and dilated apically; apical dilatation of the distal aedeagus segment with a large membraneous lobe dorsally; and R S vein of forewing apically bent towards anterior wing margin, medially straight or bent anteriad. According to this definition, the C. vicina group includes eight species in the Palaearctic Region and south Africa (all associated with Tamarix spp. ): C. bidentata Burckhardt, 1988 ; C. conspurcata Loginova, 1960 ; C. forficulata Li, 2011 ; C. linzensis Li, 2011 ; C. loginovae Baeva, 1963 ; C. namibiensis Hollis, 1974 ; C. turanica Loginova, 1974 ; and C. vicina Loginova, 1960 ( LOGINOVA 1974, BURCKHARDT 1988, LI 2011). The shape of the male paramere of C. dioscoridis sp. nov. in lateral view which is relatively slender with an elongate apical posterior lobe, is reminiscent of C. forficulata and C. linzensis from China which, however, have much shorter pterostigma on forewing ( LI 2011), and C. vicina distributed in the Caucasus, Central Asia and China which differs in the transparent forewing membrane and shorter anterior lobes of vertex ( LOGINOVA 1960, 1974). The milky white, opaque forewing membrane of C. dioscoridis sp. nov. is similar to C. loginovae from Central Asia which, however, differs from C. dioscoridis sp. nov. in its clavate paramere and reduced pterostigma ( LOGINOVA 1974). Colposcenia bidentata , C. conspurcata and C. turanica differ from C. dioscoridis sp. nov. in the shape of the paramere which is robust and clavate in the first two species and bearing a dorsally produced triangular apex in C. turanica (LOGINOVA 1974, BURCKHARDT 1988). Colposcenia namibiensis from Namibia is distinct, besides the shape of the paramere, in the very short Cu vein of forewing which branches acutely, the long antennae with rhinaria missing on segments 5 and 7, and the short female terminalia with proctiger dorsally convex ( HOLLIS 1974, BURCKHARDT 1988).
Etymology. Derived from “Διοσκουρίδα” (= Dioscorida), the ancient Greek name for the island of Socotra; noun in genitive case standing in apposition.
Host plant. Tamarix nilotica (Ehrenb.) Bunge (Tamaricaceae) .
Occurrence in Socotra. Colposcenia dioscoridis sp. nov. has been found on both the northern and southern coast of Socotra. Its host plant, Tamarix nilotica , is relatively common on the island at low altitudes, forming small thickets particularly on higher sand dunes and in salt marshes ( MILLER & MORRIS 2004, BROWN & MIES 2012), near fresh water springs ( Figs 4, 5 View Figs 1–5 ) and mouths of wadis, and is locally found also in seepage areas on limestone cliffs (Deiqab cave in Noged plain).
Distribution. So far only known from Socotra. The host plant, Tamarix nilotica , is widely distributed in coastal and arid areas of eastern Africa, Arabia and the Levant ( HASSLER 2014).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Psylloidea |
|
Family |
|
|
SubFamily |
Aphalarinae |
|
Genus |