Catenicella paradoxa, Rosso, 2009
|
publication ID |
https://doi.org/ 10.1080/00222930903089977 |
|
persistent identifier |
https://treatment.plazi.org/id/5B5A1F03-FFBD-7668-DB7B-F9BDFBF1FB4A |
|
treatment provided by |
Felipe |
|
scientific name |
Catenicella paradoxa |
| status |
sp. nov. |
Catenicella paradoxa View in CoL sp. nov.
( Figures 2–5 View Figure 2 View Figure 3 View Figure 4 View Figure 5 )
Material
Holotype. A fertile erect colony on the articulate corallinales Jania rubens (Linnaeus) Lamouroux, PMC B 17a. 5 July 2006.
Paratypes. Three sterile and one fertile colonies from the same sample, all growing on the green alga Flabellia petiolata, PMC B 17b. 5 July 2006.
Etymology
From paradoxon = strange, referring to the unexpected inferred ancestrula and colony basal organization.
Description
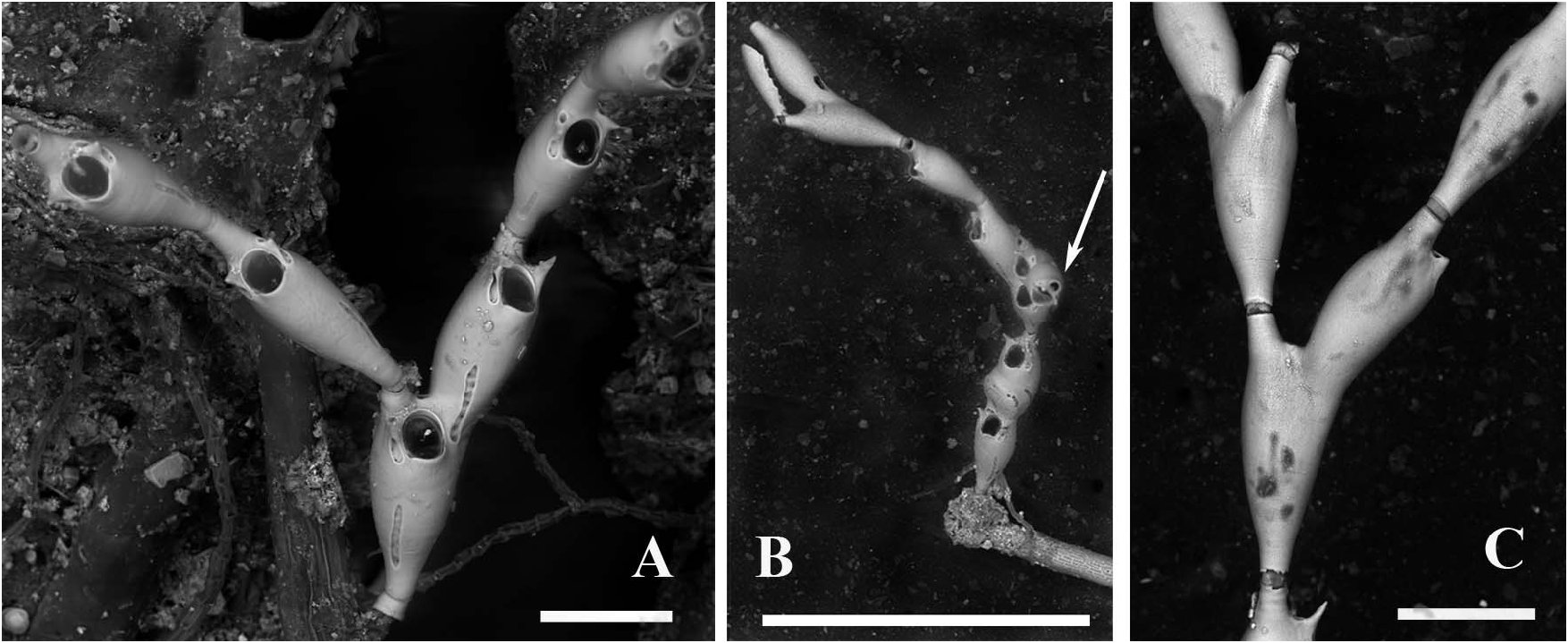
Colony erect and jointed, translucent white, poorly calcified and delicate, flexible and small, not exceeding a dozen zooids and 2–3 mm in height, poorly branching and articulated ( Figure 2A–C View Figure 2 ). Branches consisting of a few articulated segments each comprising one or two lightly calcified, vitreous and transparent zooids, all facing the same way.
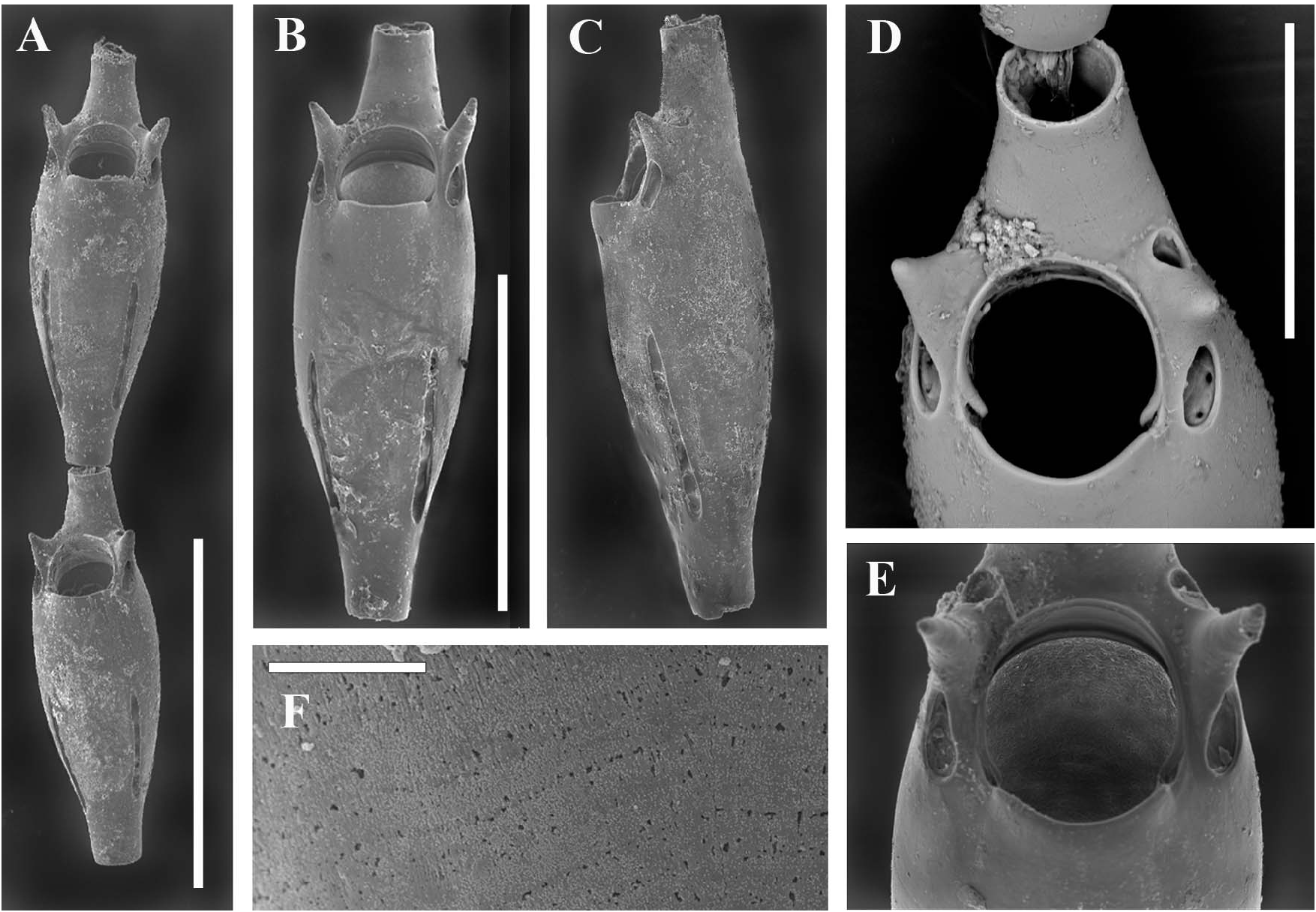
Unizooidal segments ( Figure 3A–C View Figure 3 ), consisting exclusively of non-reproductive zooids, slender and spindle-shaped, tapered proximally, 350–390 µm long, reaching 140 µm wide in their mid-distal enlarged part, ending in a dorsally placed, distal tubule (80–90 µm in diameter) for the next articulating segment. Frontal wall a smooth gymnocyst showing faint transverse growth lines (seemingly resulting from alternating increments with high and low organic contents), visible only at high magnifications ( Figure 3D View Figure 3 ). Tiny, sparse punctations are visible only in transmitted light. Primary orifice ( Figure 3E,F View Figure 3 ) terminal and slightly longer (60 µm) than wide (65–70 µm), spineless, inclined distally where a narrow distal shelf develops, with a slightly protruding concave proximal lip laterally marked by shallow indentations. Lateral condyles small but stout. Peristome developed only distally as a thin raised rim. Oral avicularia lacking. Two 40–50-µm long conical-to-spiniform lateral processes flank the distal portion of the orifice, each located between a drop-shaped (infrascapular) pore chamber facing proximo-laterally and a smaller, more rounded (suprascapular) one facing distally. Both lateral pore chambers include three or four relatively large communication pores. Lateral pore chambers marginal but visible in frontal view, proximally located and not passing beyond the midline, lightly diverging and slit-like, about 150 µm long, bearing a dozen communication pores in two roughly alternating series. Subsequent segments are often separated by a short collar-like mineralized element between two closely spaced articulation joints.
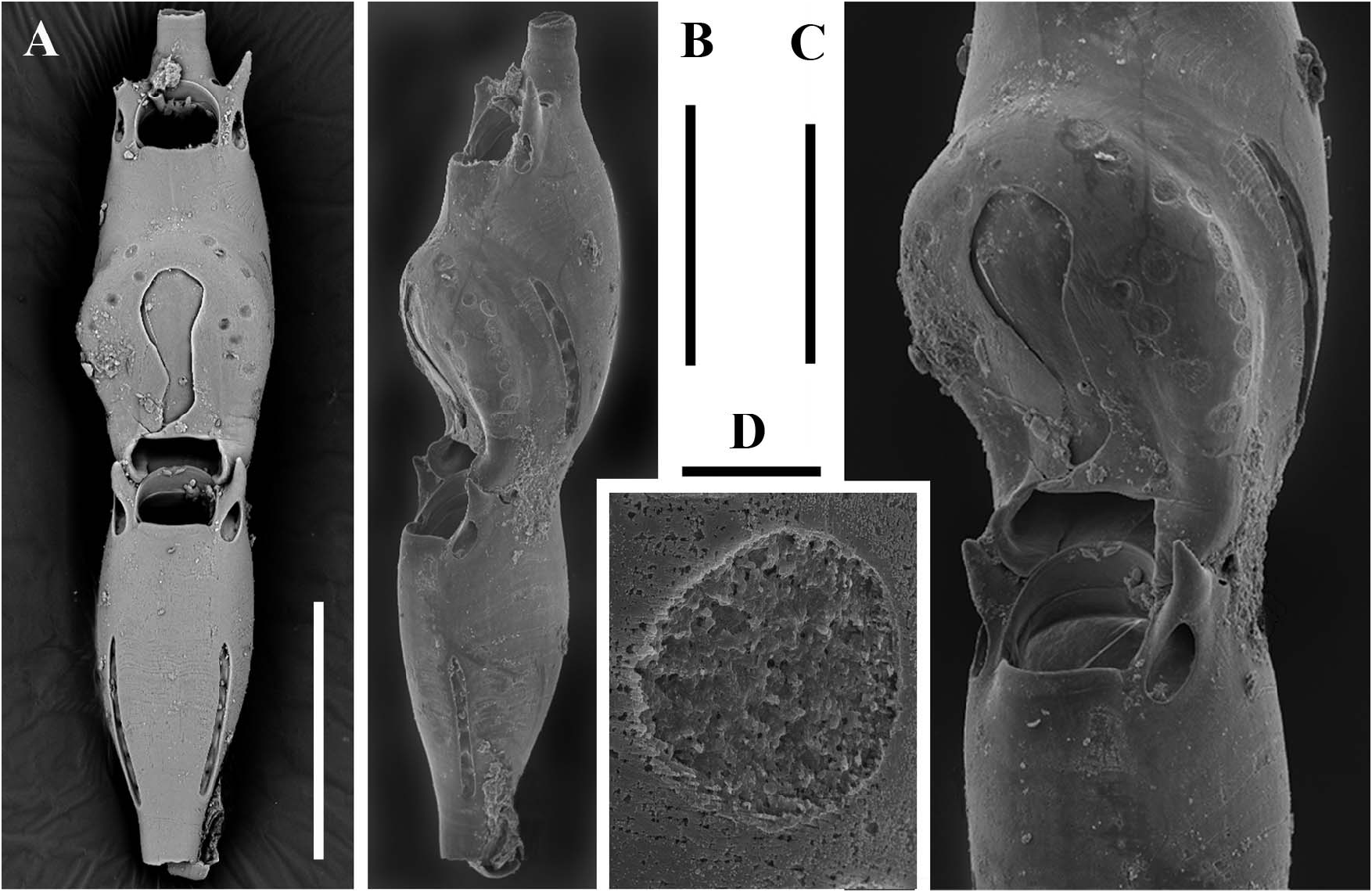
Sterile bizooidal segments ( Figures 2A–C View Figure 2 , 4A View Figure 4 ) consisting of zooids slightly shorter and less slender than those forming the unizooidal segments. Basal zooid budding a distolateral non-articulated zooid, seemingly replacing one of the oral spiniform processes. Daughter zooid originating the new branch diverging at about 30–35°, slightly tapered and narrow-based, the fusion zone restricted to the distal portion of the parental zooid.
Fertile segments bizooidal ( Figures 2B View Figure 2 , 4A–C View Figure 4 , 5D,E View Figure 5 ) with zooids aligned, each slightly shorter than those from unizooidal segments. Ovicells pouch-shaped, conspicuous, longer than wide (200–210 by 150–160 µm), embedded in the distal zooid, distally and laterally swollen and flat frontally. Calcified ectoecium with a window exposing a large drop-shaped to irregularly lobate longitudinal area of lightly calcified entoecium, and sculptured with a discontinuous peripheral row of relatively large shallow pits and a further dozen scattered on the frontal shield. Ovicell orifice transversely squeezed and subrectangular ( Figures 4A,C View Figure 4 ) located immediately distal to the zooidal orifice, not closed by the operculum. Orifice and orificial processes of fertile zooids as for the sterile ones, but lateral pore chambers of the zooid in which the ovicell is partly embedded curved to border the ovicell outline.
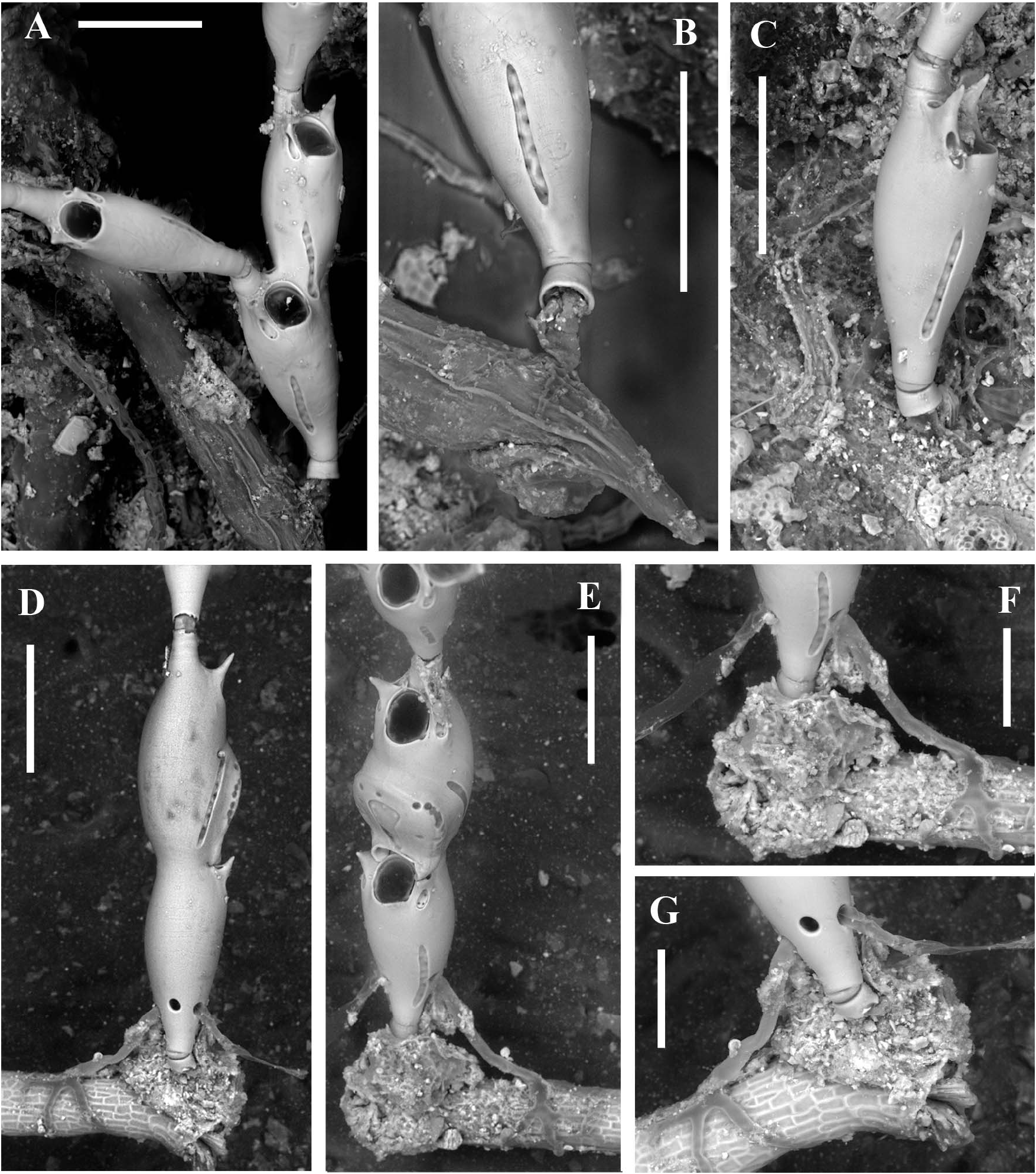
Attachment to the substratum seemingly through a short basal chitinous tubule ( Figure 5A–C View Figure 5 ), about 40 µm in diameter and up to 80 µm in length, seemingly extending into probably two short, distally tapering, thread-like encrusting projections ( Figure 5C View Figure 5 ). The chitinous tubule is partly protected by a mineralized sheet-like cover some 30 µm high slightly enlarging towards the base where the diameter does not exceed 50 µm. Such a basal annulus is followed by a second calcified element no more than 15–20 µm high, proximally tapering and partly concealed within the basal one. In the largest observed specimen (holotype) attachment is reinforced by rhizoids emerging from round pores in the proximal part of the basal autozooid ( Figure 5D–G View Figure 5 ). Rhizooids slender, about 300–500 µm long and 20 µm in diameter, adhering to the substratum with their irregularly branching ends. Two colonies encrusting F. petiolata were apparently connected to chitinous encrusting tubes, about 150 µm in diameter, albeit wrinkled after desiccation ( Figure 5A,B View Figure 5 ). Nevertheless, it was not clear whether the basal attachment tubules of C. paradoxa sp. nov. branched from the thick encrusting tube or not, because of the heavy microbial and calcareous algal mat ( Figure 5C View Figure 5 ). In contrast, the holotype specimen was attached only through rhizooids emanating from the proximal portion of the basal zooid ( Figure 5D–G View Figure 5 ).
The first recognized basal zooid is either a monozooidal segment (two instances: see Figure 5C View Figure 5 ), or the basal autozooid in a sterile bizooidal segment (one instance: see Figures 2A View Figure 2 , 5A View Figure 5 ), or the ovicellate zooid of a fertile bizooidal segment (two instances: see Figures 2B View Figure 2 , 5D,E View Figure 5 ).
Remarks
Catenicella paradoxa View in CoL sp. nov. is distinctive within the genus because of its small, slen- der zooids with short lateral pore chambers, the linear bizooidal segments and the peculiar ovicell sculpture, the lack of avicularia and the presence of spiniform processes lateral to the orifice. The last feature is reminiscent of C. contei View in CoL , the type of which has been lost (d’Hondt, personal communication in 2007). Nevertheless, the figures of Savigny (1810–11) and descriptions of Audouin (1826) (fide d’Hondt 2006) suggest that C. contei View in CoL from the Red Sea (?) differs in the general zooidal outline including the sub-terminal constriction and the lateral projection of the oral processes. Furthermore, as pointed out by Norman (1909), it lacks both frontal and lateral oral pore chambers. Despite this, some zooids near the colony base clearly exhibit a proximal rootlet pore on the frontal wall. “ Catenicella contei View in CoL ” from the eastern Atlantic, as described and figured by Norman (1909) and Waters (1909), more closely resembles C. paradoxa View in CoL sp. nov. in its zooidal outline but possesses very long frontal pore chambers extending up to the orifice, apparently lacks a lateral oral pore chamber, and has particularly long, laterally projecting oral processes ( Waters 1909). Some affinities are obvious also with “ C. contei View in CoL ” from the west Atlantic, as figured by Winston (1982) but again lateral spiniform processes are very long in these specimens, which also have globose zooids suddenly and markedly tapered proximally and fertile multizooidal segments including up to four aligned zooids and three ovicells at least in some distal branches of large colonies. The presence of ovicells in multizooidal (usually trizooidal) bifurcating segments is a character also depicted by Norman (1909) and noted by Gordon (1984) for distinguishing “ C. contei View in CoL ” from C. elegans View in CoL , which has ovicells in bizooidal segments like C. paradoxa View in CoL sp. nov. Finally, C. paradoxa View in CoL sp. nov. differs from all of the above reported figured specimens of “ C. contei View in CoL ” because the second zooids are in bifurcating bizooidal segments, not tapered proximally but parallel-sided and connected to the parental zooid at least for their distal halves. In contrast, C. paradoxa View in CoL sp. nov. stands out from “ C. contei View in CoL ” and most Catenicella species as the zooids taper slightly proximally at bifurcations and fuse with parental zooids through a narrow zone, a feature somewhat reminiscent of the bifurcation outline of C. marceli Gluhak, Lewis and Popijac, 2007 View in CoL , recently described from Taiwan ( Gluhak et al. 2007). This last character seems to be of some importance in species identification because it has been used, together with the presence of other important features (coastal frontal shield and large and shallow frontal pore chambers), to distinguish Talivittaticella Gordon and d’Hondt, 1985 View in CoL , a genus closely related to Catenicella View in CoL , by Gordon and d’Hondt (1985: 19).
Finally, C. paradoxa View in CoL sp. nov. at first sight also superficially resembles C. elegans View in CoL , which differs mostly in the presence of lateral oral avicularia in place of spiniform processes, and also in the long frontal pore chambers, the more swollen zooids, the ovicell with a round central lacuna and the wide fusion between zooids at bifurcations.
The species is placed within Catenicella de Blainville, 1834 to maintain nomenclatural stability and following Gordon (1984: 66) who considers this genus to be a valid senior synonym with priority over both Caloporella MacGillivray, 1895 and Vittaticella Maplestone, 1901 . Catenicella contei is the first quoted species for Catenicella by de Blainville (1834) although under the name C. savignyi and with a clear reference to specimens figured by Savigny (1810–11) in the Déscription de l’Égypte ( d’Hondt 2006), and hence considered as its type species (see also Gordon 1984). Nevertheless, the description of the genus was clearly given referring to a different and completely unrelated species, namely the encrusting Hippothoa divaricata Lamouroux, 1821 , and specifically to specimens originating from the Mediterranean, consequently causing further misunderstanding about the geographical distribution of C. contei (see below).
Within the genus Catenicella some 36 fossil and/or living species were listed by Bock (2008), all sharing similar orifice morphology but different colony organization and zooidal features. Attempts to allocate these species within different generic and subgeneric entities have been made in the past, based on features such as the presence or absence of frontal pore chambers (vittae), or the number of zooids in the ovicellbearing segments, a character revealed to be highly variable within single species (see Gordon 1984, 1989a). Further features, such as the presence of lateral oral spiniform processes and compressed projecting lateral knobs in place of avicularia, or ovicell sculpture, seem more stable and could be more useful. Furthermore, as most Catenicella species are known only from rough descriptions and schematic drawings, a revision of types and materials examined by early authors is needed to define species characters and their variability using SEM analysis.
Catenicellid history from the northern hemisphere and the European area
The family Catenicellidae Busk, 1852 is mostly known from the Australasian region, where several diverse genera thrive ( Lagaaij and Cook 1973; Gordon and Braga 1994; Bock 2008). Nevertheless, according to Gordon and Braga (1994) it first appeared in the westernmost sector of the Tethys Ocean, later spreading eastward. The oldest catenicellid species is known from the Maastrichtian of Jamaica and Late Paleocene (Thanetian) taxa occur in Europe. They belong to the genus Caberoides , namely Caberoides canaliculata Canù, 1908 and Caberoides rockallensis Gordon and Braga, 1994 , respectively found in northwestern France and on the Rockall plateau (northeast Atlantic). The family diversified during the Eocene. Several species with articulate multizooidal biserial segments are known from that time, belonging to four genera: Caberoides canaliculata Canù, 1908 and Caberoides grignonensis Canù, 1908 , Ditaxipora luteciana (Canù, 1913) and Ditaxipora labiata (Canù, 1910) , Ditaxiporina granulosa, Canù, 1908 , and Ahcheethamia corniculata Gordon and Braga, 1994 . During the Priabonian and the Oligocene new biserial species appeared within the same genera, namely Caberoides continua (Waters, 1891) , Ditaxipora pannonensis Braga, 1980 and Ditaxiporina septentrionalis (Waters, 1891) , all belonging to the subfamily Ditaxiporinae , which did not survive into the Miocene in the area. From that time on catenicellids were mostly represented by uniserial species. Costatimorpha algella from the Priabonian of Austria ( Zagoršek 2003) is the first known European uniserial catenicellid with a field of costal spines. The genus Catenicella appeared first in the European area during the Middle Eocene (Lutetian) in the Paris Basin ( Lagaaij and Cook 1973; as Vittaticella sp. ). Species belonging to this genus, though not yet described, existed during the Late Eocene and the Lower Oligocene in north Italy ( Braga 2008: fig. 15a,b, as Catenicellid sp.), the Late Oligocene and the Middle Miocene in France ( Lagaaij and Cook 1973), and the Lower Miocene in north Italy (Braga, personal communication 2007). Catenicella elegans has been recently reported ( Moissette et al. 2006, 2007) from the Badenian of Hungary. This uniserial species with uni- to bizooidal segments seemingly persisted in the European area through the Messinian (Gulf of Gabes: southern Mediterranean: Moissette 1997), the Pliocene (northern Europe: Lagaaij 1968; Cadée 1973; Pouyet 1997) and the Late Pliocene (Rhodes, Mediterranean: Lagaaij and Cook 1973), although there are some doubts about the conspecificity of the Gabes specimens (cf. Moissette 1997: pl. 3, figs 9, 14). From cores of the Messinian sediments in the Gulf of Gabes, the uniserial unizooidal Vasignyella otophora (Kirkpatrick, 1890) is also known ( Moissette 1997). A diversification phase occurred in the Early Pliocene, with Cornuticella cornuta ( Busk, 1852) and Scalicella umbonata ( Busk, 1852) recorded from northern France ( Pouyet 1997) in addition to C. elegans . Finally, at least three other Catenicella species occur in the Middle Pliocene (less than 3 million years ago) sediments cropping out near Forlì, northern Italy ( Neviani 1928), namely Catenicella zancheri ( Neviani, 1928) , Catenicella cipollai ( Neviani, 1928) and Catenicella capitiscollis ( Neviani, 1928) , differing mostly in zooidal size and shape and the presence of lateral avicularia or spiniform processes and closely related to both C. elegans and C. contei . The specimens of the Neviani collection were subsequently revised and redescribed by Annoscia (1966) and never recorded elsewhere. Only recently, several segments seemingly belonging to at least one of these species have been found (Rosso, personal observations) in Upper Pliocene to Lower Pleistocene sediments cropping out near Castroreale (northeastern Sicily) and interpreted as deposited in shallow-water settings ( Messina et al. 2007).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Catenicella paradoxa
| Rosso, Antonietta 2009 |
Catenicella paradoxa
| Rosso 2009 |
C. paradoxa
| Rosso 2009 |
C. paradoxa
| Rosso 2009 |
C. paradoxa
| Rosso 2009 |
C. paradoxa
| Rosso 2009 |
C. paradoxa
| Rosso 2009 |
C. marceli
| Gluhak, Lewis and Popijac 2007 |
Talivittaticella Gordon and d’Hondt, 1985
| Gordon and d'Hondt 1985 |
Catenicella
| de Blainville 1830 |