Asperaxis karenae, Alderslade, 2006
|
publication ID |
https://doi.org/ 10.5281/zenodo.2646378 |
|
publication LSID |
lsid:zoobank.org:pub:9981684F-AC10-421F-9944-B3EDECF89993 |
|
persistent identifier |
https://treatment.plazi.org/id/03D887FF-D97B-FFB6-FE86-FECFFDB6369E |
|
treatment provided by |
Plazi |
|
scientific name |
Asperaxis karenae |
| status |
sp. nov. |
Asperaxis karenae View in CoL new species ( Figs 1–15 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 View FIGURE 11 View FIGURE 12 View FIGURE 13 View FIGURE 14 View FIGURE 15 )
Material examined. Holotype: NTM C14986, Mundy Island , Bathurst Channel, Port Davey, Tasmania, Australia, depth 4–6 m, K. GowlettHolmes, 13 April 2002.
Paratypes: NTM C13575, same data as holotype ; NTM C14987, Sarah Island , Bathurst Channel, Port Davey, depth 8 m, G. Edgar, 20 March 1998 ; SAM H1397 View Materials , Sarah Island , Bathurst Channel, Port Davey, depth 5–12 m, rock slope to mud bottom, WEB station 1, K. GowlettHolmes, W. Zeidler, F.A. Bavendam, 3 April 1993 ; SAM H1398 View Materials , Schooner Cove , off Forrester Point , Bathurst Channel, Port Davey, steep wall, mud bottom, depth 3–18 m, WEB station 2, K. GowlettHolmes, W. Zeidler, F.A. Bavendam, 2 April 1993 ; SAM H1399 View Materials , same data ; SAM H1400 View Materials , Cathedral Cave , Waterfall Bay , Tasman Peninsula, Tasmania, depth 15–18 m, growing over phidoloporid bryozoan on rock wall, K. GowlettHolmes, 29 October 1994 ; SAM H1401 View Materials , Patersons Arch , Waterfall Bay, Tasman Peninsular, depth 10–13 m, on rock wall, K. GowlettHolmes, 17 September 1995 .
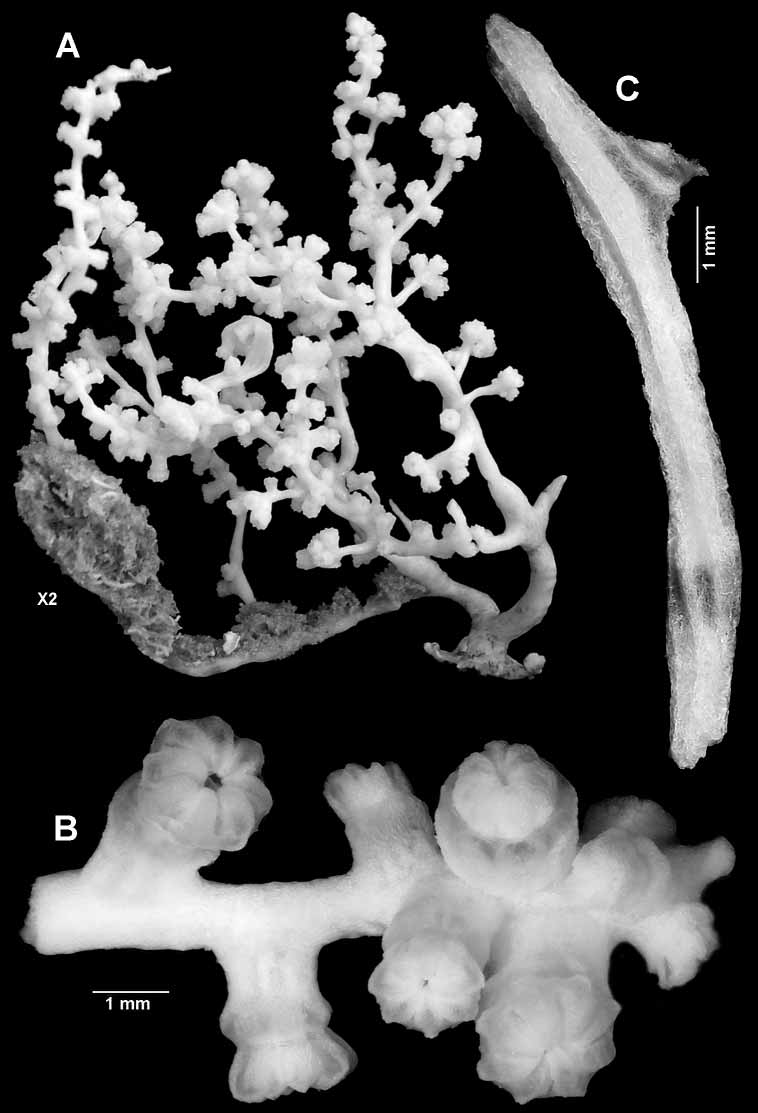
Description. The holotype is a fragile, bushy, rather sparsely branched, 60 mm high colony ( Figs 1–2A View FIGURE 1 View FIGURE 2 ). The very short, slightly flattened main stem is of uneven thickness with broadest diameter of 4.4 mm. It arises from a small 13 x 6 mm holdfast and immediately divides into two major branches, 2.2 mm and 2.4 mm in diameter respectively. One of these gives rise to a lateral branch that is overgrown by sponge for most of its length.
Branching in the colony is irregularly lateral to the third order, with terminal twig lengths varying from about 5–35 mm in length and 0.75–1.6 mm diameter. Nearly every twig terminates in a cluster of polyps ( Fig. 2A, B View FIGURE 2 ). Branching may arise at an acute angle or is more or less perpendicular, and the branches are sinuous.
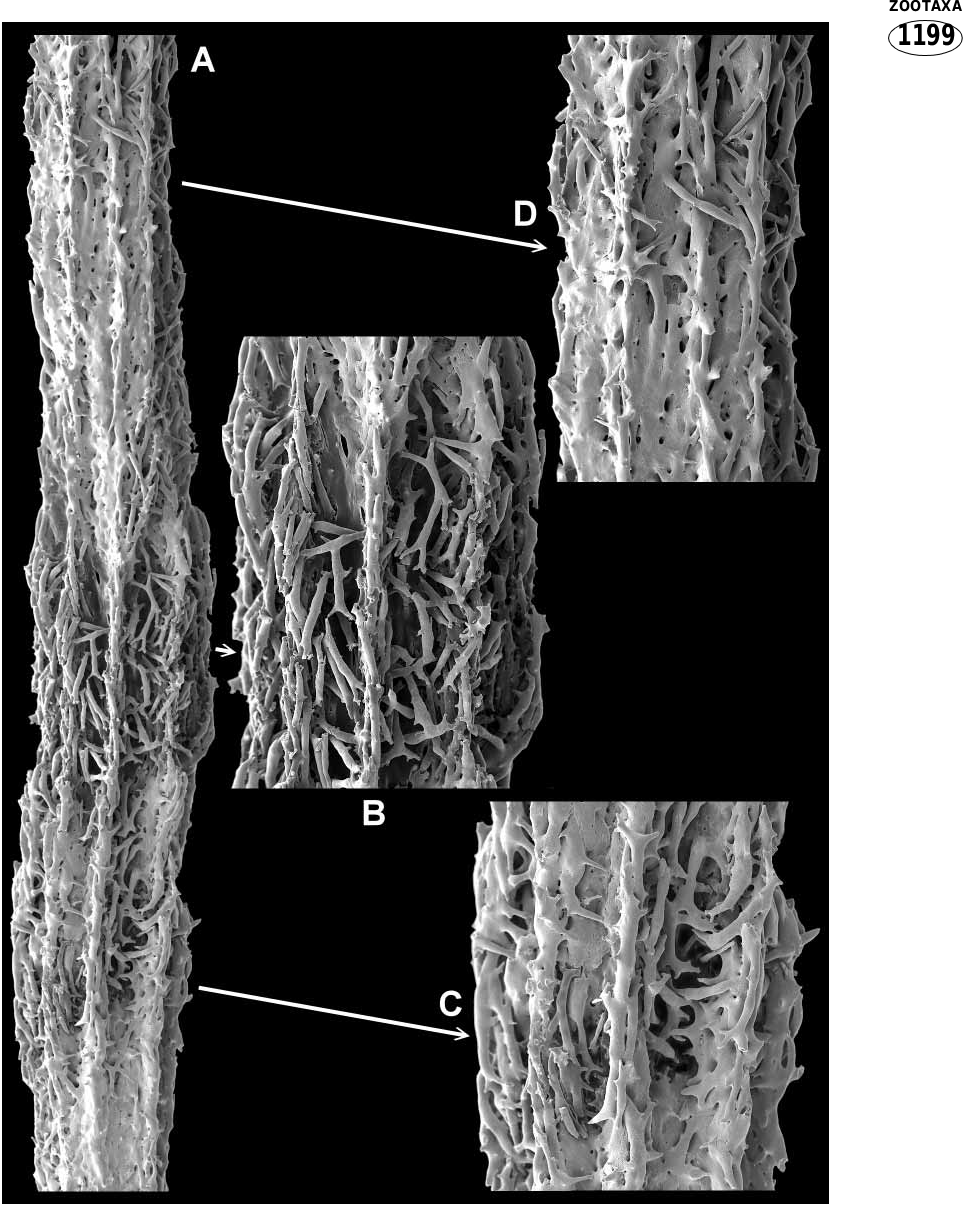
The axis consists of hard calcareous internodes and softer organic nodes, both of which incorporate sclerites ( Figs 2C View FIGURE 2 , 3 View FIGURE 3 ). The internodes are formed from sclerites wholly or partially embedded in a matrix of subhedral calcite rods ( Fig. 5 View FIGURE 5 ). In some portions of the internodes, apparent imperfect or ongoing infilling with calcareous matrix has left the axis penetrated by numerous holes or canals. The internodal sclerites are mostly stick like, sparsely tuberculated, often sinuous and often branched ( Fig. 11A View FIGURE 11 ) (and coenenchy mal sclerites are occasional included; see Fig. 6 View FIGURE 6 Ea). On the outside of the internode they are partially embedded, commonly anastomosing and usually gathered into longitudinal ridges although they may cover much of or all the surface (compare Figs 3 View FIGURE 3 & 9A View FIGURE 9 ). Large longitudinal canals run through the coenenchyme between the axial ridges, as can be seen in the thick, partially decalcified section in Fig. 4B View FIGURE 4 . The interior of the internode is partly hollow and contains a 3dimensional latticework of anastomosing tuberculate spindles that is penetrated by mesogloea ( Figs 4A View FIGURE 4 ; 9C,D View FIGURE 9 ). Figure 5A View FIGURE 5 depicts a longitudinal section through part of an internode, clearly showing part of the core lattice. The presence of embedded sclerites in the solid parts of the axis are not obvious from this aspect, as can be seen in the closeup ( Fig. 5C View FIGURE 5 ), because the calcite rods from which they are constructed blend so well with those of the matrix. They can, however, easily be seen in a crosssectional view, as depicted in Figure 9E, F View FIGURE 9 .
Internodes begin in the tips of terminal twigs as an aggregation of both free and anastomosed sclerites. Infilling of the axis with the calcite matrix increases proximally. A growing terminal internode of a paratype is shown in Fig. 6A View FIGURE 6 . The actual tip of this sample collapsed during processing with sodium hypochlorite to remove the coenenchyme. Figure 6F View FIGURE 6 shows the tip of another twig axis processed for less time; the tuberculate spindles in the centre ( Fig. 6 View FIGURE 6 Fa) were presumably destined to be incorporated into the trabecular core of the internode. Figure 6D View FIGURE 6 shows the cavity left where a small partial node has been dissolved.
Nodes may be whole (ie. completely divide a branch portion into separate internodes as in Figs 3B View FIGURE 3 ; 7E View FIGURE 7 ) or they may be partial. Partial nodes vary from those that almost completely separate internodes to those that are exceedingly small and inset into the side of an internode ( Figs 3C View FIGURE 3 ; 6D View FIGURE 6 ; 7 View FIGURE 7 A–D). There is no distinct boundary between nodes and internodes. At the juncture, the end of the internode consists of an irregular arrangement of partially embedded, protruding sclerites between which the nodal material has been deposited; figure 9B shows a very short, complete internode. Partial nodes are similar in that the solid internodal material gives way to partially embedded sclerites ( Fig. 7A,B View FIGURE 7 ). Nodal sclerites appear to be the same as the sticklike internodal forms with the addition of short rods with thickened tuberculate ends ( Fig. 7D View FIGURE 7 ). On the node surface these sclerites are often seen to been aggregated in such a way as to form continuations of the internodal sclerite ridges, and some anastomoses may occur; see Fig. 3B View FIGURE 3 (whole internode) and Fig. 3C View FIGURE 3 (partial internode). Within the node, sclerites are held in an organic matrix. The sclerites are sheathed in gorgonin, which binds them together leaving interconnected cavities containing mesogloea ( Fig. 8 View FIGURE 8 A–C).
Branches arise from large, whole or partial nodes that are mostly inconspicuous (especially on thin branches) as they are commonly more or less the same thickness as the adjoining internodes. Nodes are also present in nonbranching sections, and there may be a number of them crowded between points of bifurcation. They can be slightly swollen relative to the internodes, but this may not be reflected in a notable increase in branch thickness unless on thick basal branches. Internode length is estimated to vary from 0.75– 10.00 mm, and nodes can be at least 1.5 mm long. The range of length of axial segments is difficult to accurately ascertain because the process is destructive.
One of the reasons this colony has been selected as the holotype is that the polyps are slightly distended. The tentacles are folded over the mouth, and the polyp head generally sits just above the calyx ( Fig. 10A View FIGURE 10 ). Polyps are scattered irregularly along the branches, either singly or in groups of 2 or 3, and are gathered in terminal clusters of 4–15 ( Fig. 2B View FIGURE 2 ).
A few calyces are cylindrical but most are slightly expanded distally showing the contracted introvert between the rim and the polyp head. The largest calyx is 1.2 mm tall, 1.2 mm basal diameter, and 1.9 mm distal diameter. The largest polyp head is 1.2 mm tall and 2.0 mm across. Juvenile polyps occur here and there on branches, but are most commonly found in the terminal clusters where they can be as short as 0.4 mm.
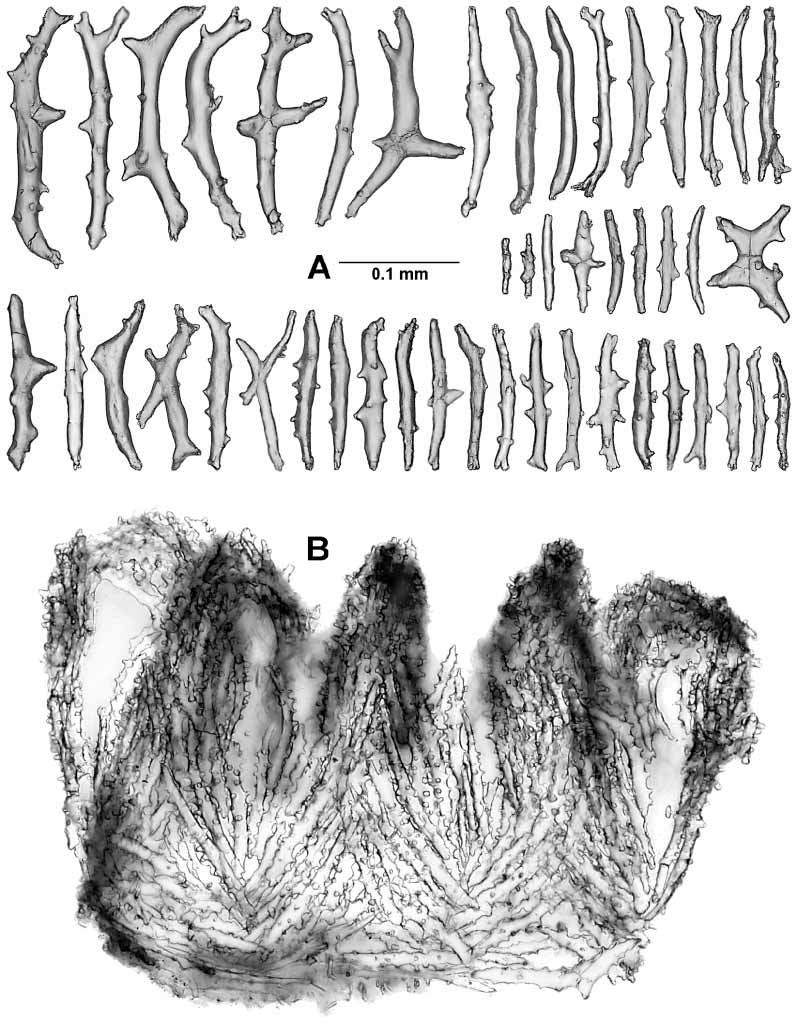
The calicular sclerites are sticks, spindles, and clubs about 0.15–0.28 mm in length ( Fig. 12 View FIGURE 12 ). The tubercles are mostly large, complex, and irregularly arranged. The sclerites are oriented longitudinally in the lower half of the calyx, becoming formed into 8 chevrons in the upper half, and forming 8 teeth on the calyx rim ( Fig. 10A View FIGURE 10 ).
The polyp head is heavily armed with numerous point sclerites ( Fig. 10A View FIGURE 10 ). At the base of each point the sclerites are more or less horizontally arranged and these combine with a small number of long bowshaped sclerites to constitute a weak collaret. Above this region the sclerites are in chevrons, becoming longitudinal as they encroach on the base of each tentacle. Between each set of points there is a group of 2–7 (usually about 2) lower, intermediate, sticklike sclerites of various sizes, and above this there are 0–2 small, upper, intermediate sclerites ( Fig. 10B View FIGURE 10 insc, 11B). The point sclerites are sticks and clubs. A few clubs ( Fig. 13 View FIGURE 13 Aa) occur in the distal part of each of the points. They have large tuber cles and branched heads and are about 0.12–0.20 mm long. The remainders of the point sclerites are flattened sticks c. 0.07–0.29 mm long with simple tubercles ( Fig. 13 View FIGURE 13 Ab). In the pharynx there are small rods about 0.036–0.066 mm long that have two medial girdles of knoblike tubercles ( Fig. 13B View FIGURE 13 ).
Along each edge of the tentacles is a single row of about 10 pinnules that are ringed with nematocyst batteries ( Fig. 10 View FIGURE 10 C–D). The aboral aspect of the tentacle is covered with two rows of transversely placed scales ( Figs 10B View FIGURE 10 , 14A View FIGURE 14 ) that are about 0.09–0.28 mm long. Proximally, the scales are crescentshaped to fit the curvature of the tentacle surface and they are complexly branched or have branched tubercles. Distally the scales become shorter and simpler in design. On the adoral face there is a single small scale associated with the base of each pinnule ( Fig. 10B View FIGURE 10 pisc, D). These scales are asymmetrical folia ceous crescents ( Fig. 14B View FIGURE 14 ), 0.09–0.16 mm long and they lie curved around the proximal side of the pinnule base. The narrow tail of the scale seems to lie on the lateral edge of the tentacle rachis, the crescent curves around the pinnule base, and the foliaceous portion lies on the oral face of the pinnule base.
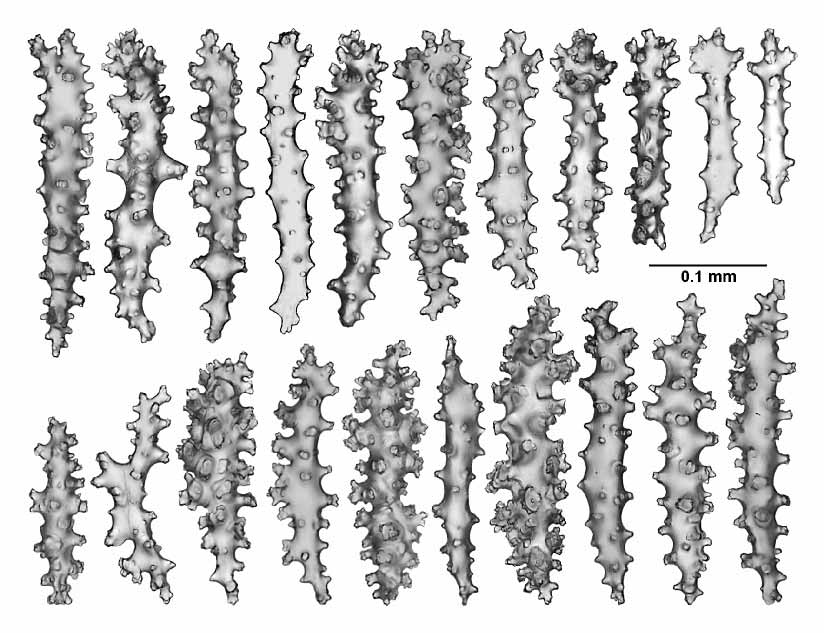
The sclerites of the twigs and branches are complexly tuberculated sticks and spindles, occasionally branched, 0.05–0.28 mm in length ( Fig. 15 View FIGURE 15 ).
Colour. Live colonies are pale pink. Preserved colonies are white to pale brown with white internodes and pale yellow nodes.
Variability: Several of the paratype lots consist of colonies or colony fragments that are noticeably thinner than the holotype.
a) Polyp density can be quite variable, with some colonies having long branches with very few polyps
b) Clusters of polyps are very common, and do not only occur at the end of terminal branches.
3) Clublike sclerites may be few or even rare in the tip of the points arrangements.
4) Several lots show signs of initial preservation in acidic formalin.
Etymology. The species is named for my good friend and colleague Karen Gowlett Holmes, Marine Collection Manager, CSIRO Tasmania, and multiaward winning underwater photographer. Karen is a regularly donator of material to the MAGNT collected during her many private expeditions.
Remarks. The species is known from two types of habitat. The following comments are those of Karen GowlettHolmes:
“Both habitats are extremely lowlight, and both have other taxa usually found in much deeper environments. In the Waterfall Bay caves, the low light is similar to about 50+ m deep, and much of the fauna is the same as that seen at this depth or deeper. The Bathurst Channel, on the other hand, appears to be an isolated water body with regards to the open ocean, and has a strong pycnocline across the mouth of the channel. The area is a drowned river valley, flooded sometime about 12,000 to 15,000 years ago, and it is likely that the current sessile fauna invaded the area at that time and has been virtually isolated for about 10,000 years. What makes the region most unusual is that the upper two to four metres of the water column is a distinct layer of virtually fresh, darkly tanninstained water (like very strong black coffee). The transition into the marine layer below is very sharp and the marine layer is very clear and very dark. The fauna of the Bathurst Channel most closely resembles that of the shelf break and upper slope, for example 80 to> 120 m depth. I therefore think the new taxon is probably a deepwater emergent species. Further evidence for this is that the new taxon is always associated with various bryozoan species and these species are also known from deep water. It occurs predominantly with Triphyllozoon floribundum (Phidoloporidae) , a fenestrate bryozoan that forms small colonies in the caves but very large colonies (up to at least 60 cm in diameter) in deeper water and in the Bathurst Channel. Sometimes the gorgonian is found with Schizoretepora tesselata (Phidoloporidae) , a poorly known fenestrate species found in the caves and the Bathurst Channel, and occasionally dredged on the shelf break. Less commonly, the new gorgonian is found associated with Idmidronea sp. (Cyclostomata: Tubuliporidae ), an undescribed, finely branching, arborescent spe cies found in the caves, on rock walls, and common out to the shelf break. On one occasion the new gorgonian was found with Adeonellopsis sp. ( Adeonidae ), a pos sibly undescribed branching species that is one of the dominant species found on the outer shelf, shelf break, and upper slope, and is common in dark areas in the caves and in the Bathurst Channel.
Bryozoans in this part of the world are rarely overgrown by anything (at least for long), as they produce deterrent secondary metabolites and have other means of defence as well. It may be worth noting, therefore, that it is quite common to find small colonies of Mopsella zimmeri growing on Triphyllozoon munitum in South Australia, so perhaps some groups of melithaeids have an affinity with phidol oporids in particular.”
Comparative Comments. Although the axis of the new genus has obvious morphological differences to that found in a nominal melithaeid genus such as Acabaria or Melithaea , the basic constructional scheme is very similar.
One of the obvious differences is that in current nominal genera the nodeinternode articulation is relatively well defined. The node of a species of Acabaria is depicted in Fig. 16A View FIGURE 16 . The overlying coenenchyme has been removed, revealing the typical melithaeid axial sclerites partially cemented together on the internodal surface ( Fig. 16B, C View FIGURE 16 ) and forming a 3dimensional meshwork in the node ( Fig. 16D View FIGURE 16 ). An enlargement of the region where the hard internode material abuts the softer nodal substance is shown in part E of that figure. Across the centre of this image, part of a latticework can be seen where sclerites are anastomosing or linked by calcite bridges ( Fig. 16 View FIGURE 16 Ea). If all the soft nodal material and loose sclerites are removed from the end of an internode, this latticework is shown to be covering its conoid end in a shallow layer ( Figs 17B, C View FIGURE 17 ; 18D View FIGURE 18 ). The sclerites in the rounded tip of the internode, however, are different ( Fig. 17 View FIGURE 17 Aa). These are larger spindles with a number of rounded tubercles, somewhat similar to those that can be found in the axis that is forming in the tips of Asperaxis twigs, which presumably become incorporated into the latticework of the internodal core. The formative melithaeinid axis appears to be similar to that of Asperaxis . Figure 18A View FIGURE 18 depicts a decorticated twig tip of a Melithaea species. As in Asperaxis , an internode begins as an aggregation of loosely bound tuberculate spindles ( Fig. 18 View FIGURE 18 Ba), the rodshaped sclerites by which the melithaeid axis has usually been characterised occurring only on the outside ( Fig. 18 View FIGURE 18 Ab, Bb). Figure 18C View FIGURE 18 shows the surface of an older internode; cavities are still present where the calcite infilling is not yet complete. In the early stages of sclerite fusion, an internode is mainly just a cylindrical latticework (the future core), with a layer of small, loose, rodlike sclerites on the outside ( Fig. 18E View FIGURE 18 ). As the internode ages, the core becomes surrounded by large numbers of these rods embedded in a matrix of subhedral calcite rods ( Fig. 17 View FIGURE 17 D–F). The core continues right into the tip of the conoid end of the internode ( Fig. 18D View FIGURE 18 ) and, according to Koelliker (1865, pl. XV, fig. 8), the mesogloea of the core (his “Centralstrang”) is continuous from internode to internode through the node.
Axes from specimens having the characters of classical species of Melithaea , Mopsella , and Acabaria were examined in the course of this research and all were found to have the same basic construction. Additionally, because the sparsely tuberculate axial sclerites of Asperaxis (eg. Fig. 9A View FIGURE 9 ) have similarities to those in the axes of Subergorgia and Annella , species of these genera were also examined. The axis of Annella reticulata ( Ellis & Solander, 1786) and that of Subergorgia suberosa ( Pallas, 1766) are illustrated in figures 19 and 20 respectively. The presence of free tuberculate sclerites in a subergorgiid axis (sclerites like those in the outer coenenchyme) is not well documented despite being discovered by Koelliker 140 years ago (1865, p. 144 and pl. XV, fig. 2). Indeed, it was overlooked by LópezGonzález and Gili (2001, p. 122), who used the presence of these sclerites in the axis of the new genus Rosgorgia as a major character distinguishing that genus from Annella and Subergorgia . Fabricius and Alderslade followed suit pending further investigation (2001, p. 56). In the several samples of Annella and Subergorgia that were analysed for this paper, tuberculate sclerites were commonly found in the centre of the axes, but they are not restricted to this region ( Figs 19B View FIGURE 19 , 20B View FIGURE 20 ). However, the simple, nonanastomosing spindles (with or without a few terminal tubercles) reported and figured by a few authors (eg. Aurivillius 1931, p. 21, fig 2 (centre); Bayer 1956, p. F198, 2c) do seem to be restricted to the centre of the axes ( Figs 19B,C View FIGURE 19 , 20B,C View FIGURE 20 ). At least in the few samples examined, this central section is certainly not composed solely of tuberculate coenenchymalstyle sclerites as stated by Kinoshita (1910, p. 224–225) and seemingly implied by Muzik and Wainwright (1977, p. 321).
Acknowledgments
My thanks to Graham Edgar and Karen GowlettHolmes for donating specimens of the new taxon, and additionally to Karen for sharing her knowledge of the new taxon’s habitats and associations. Thanks also to Thierry Laperousaz for the loan of material from the South Australian Museum, and to Ellie Haywood, Charles Darwin University, for providing the MalloryHeidenhain’s stain. I am also very grateful to my friends and colleagues Leen van Ofwegen (Nationaal Natuurhistorisch Museum, Leiden), Suzanne Horner (MAGNT), Gary Williams (California Academy of Sciences, San Francisco), and Manfred Grasshoff (Naturmuseum Senckenberg, Frankfurt) for critically reading the manuscript.
References
Alderslade, P. (2002) A new soft coral genus (Coelenterata: Octocorallia) from Palau. The Beagle, Records of the Museums and Art Galleries of the Northern Territory, 18, 1–8.
Alderslade, P. (2003) A new genus and species of soft coral (Octocorallia: Alcyonacea) from Lord Howe Island, Australia. Zoologische Verhandelingen, 345, 19–29.
Aurivillius, M. (1931) The gorgonians from Dr. Sixten Bock’s expedition to Japan and Bonin Islands 1914. Kungliga Svenska Vetenskapsakademiens Handlingar, (3) 9 (4), 1–337.
Bayer, F.M. (1956) Octocorallia. In: Moore, R.C. (Ed) Treatise on Invertebrate Paleontology Part F. Coelenterata. Geological Society of America and University of Kansas Press. Lawrence, Kansas. Pp 163–231.
Bayer, F.M. (1981) Key to the genera of octocorallia exclusive of the Pennatulacea (Coelenterata: Anthozoa), with diagnosis of new taxa. Proceedings of the Biological Society of Washington, 94, 901–947.
Ellis, J. & Solander, D. (1786) The natural history of many curious and uncommon zoophytes, collected from various parts of the globe by the late JOHN ELLIS…Systematically arranged and described by the late DANIEL SOLANDER. Benjamin White & Son, London, 13 + 209 pp., 63 pls.
Fabricius, C. & Alderslade, P. (2001) Soft Corals and Sea fans: a comprehensive guide to the tropical shallow water genera of the CentralWest Pacific, the Indian Ocean and the Red Sea. Australian Institute of Marine Science, Townsville, 264 pp.
Grasshoff, M. (1999) The shallow water gorgonians of New Caledonia and adjacent islands (Coelenterata: Octocorallia). Senckenbergiana Biologica, 78, 1–121.
Grasshoff, M. (2000) The gorgonians of the Sinai Coast and the Strait of Gubal, Red Sea (Coelenterata: Octocorallia). Courier Forschungsinstitut Senckenberg, 224, 1–125.
Grasshoff, M. & Bargibant, G. (2001) Coral Reef Gorgonians of New Caledonia. Les Gorgones des récifs coralliens de NouvelleCalédonie. Éditions de L’IRD, Institute de Recherche Pour le Développement, Collection Faune et Tropical 38, Paris, 335 pp.
Gray, E. (1870) Catalogue of lithophytes or stony corals in the British Museum. British Museum, London, 4 + 51 pp.
Hickson, S. (1937) The family Melitodidae. Transactions of the Zoological Society of London, 23, 73–212.
Kinoshita, K. (1910) On the Keroeididae, a new family of Gorgonacea, and some notes on the Subergorgiidae. Annotationes Zoological Japonenses, 7, 229–230, pl. 6.
Koelliker, A. (1865) Icones histiologicae oder Atlasder vergleichenden Gewebelehre; erstes Heft. Zweite Abtheilung. Der feiner Bau der höheren Thiere. Die Bindesubstanz der Coelenteraten. Wilhelm Engelman, Leipzig, pp. 87–181, pls 1019.
LópezGonzález, P. & Gili, JP. (2001) Rosgorgia inexplicata, new genus and species of Subergorgiidae (Cnidaria, Octocorallia) from the Antarctic Peninsula. Polar Biology, 24, 122–126.
Milne Edwards, H. & Haime, J. (1857) Histoire naturelle des coralliaires ou polypes proprement dits, Vol. 1. Paris, pp. i–xxxiv + 1–326, 8 pls.
Muzik, K. & Wainwright, S. (1977) Morphology and habitat of five Fijian sea fans. Bulletin of Marine Science, 27, 308–337.
Nutting, C. (1911) The Gorgonacea of the Siboga Expedition. VIII. The Scleraxonia. SibogaExpeditie Monographie 13b [=Livr. 57], Leiden, pp. 1–62, pls 1–12.
Pallas, P.S. (1766) Elenchus zoophytorum sistens generum adumbrationes generaliores et specierum cognitarum succinctas descriptiones cum selectis auctorum synonymis. Petrum van Cleef, Hagae Comitum, 16 + 28 + 451 pp.
Ridley, S.O. (1884) Alcyonaria. In: Report on the zoological collections made in the IndoPacific Ocean during the voyages of the H.M.S. “Alert” 18812, British Museum, London, pp. 327– 365, 578–581, pls 36–38.
Wright, E.P. & Studer, T. (1889) Report on the Alcyonaria collected by H.M.S. Challenger during the years 1873–1876. Report on the Scientific Results of the “Challenger ”, Zoology, 31, London, 27 + 314 pp., 49 pls.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |