Mycale (Arenochalina) mirabilis ( Von Lendenfeld, 1887 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4912.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:9536C1CF-4AEF-47F8-959B-48CD7A5392D8 |
|
DOI |
https://doi.org/10.5281/zenodo.4473184 |
|
persistent identifier |
https://treatment.plazi.org/id/361087A7-FFF6-FF95-55AB-FEEF5223CC9C |
|
treatment provided by |
Plazi |
|
scientific name |
Mycale (Arenochalina) mirabilis ( Von Lendenfeld, 1887 ) |
| status |
|
Mycale (Arenochalina) mirabilis ( Von Lendenfeld, 1887) View in CoL
Arenochalina mirabilis Von Lendenfeld, 1887: 821 View in CoL , pl. XXVI fig. 70, pl. XXVII fig. 28; Von Lendenfeld 1888: 103; Whitelegge 1902: 212–213; Pulitzer-Finali 1982a: 100, fig. 12.
Esperella spongiosa Dendy, 1896: 16 View in CoL .
? Esperella crassa Dendy, 1896: 17 View in CoL .
? Esperella rara Dendy, 1896:18 .
Mycale (Arenochalina) mirabilis View in CoL ; Hallmann 1912: 252 (footnote); Carpay 1986: 32; Wiedenmayer 1989: 84, pl. 9 figs 8–10, 13, pl. 10 figs 1–2, pl. 29 figs 1–4, text figs 53–56 (see also there for possible synonymies and extensive description); Van Soest & Hajdu 2002: 677.
Naviculina mirabilis View in CoL ; Hooper & Wiedenmayer 1994: 293.
Mycale mirabilis View in CoL ; Erpenbeck et al. 2016: Supplementary Data 2, 28S and COI trees.
Material examined. ZMA.Por.P.12173, Australia, Tasmania, Eaglehawk Neck, coll. M.C, Carpay, slide made from single erect specimen of 30 cm long, kept at Tasmanian Museum, reg.nr. TM 85.
Summary description. Sponges are massive, erect club-shaped to lobate, or encrusting, with coarsely spinous surface. Color in life brownish yellow. On deck, the sponge becomes slimy and looses much of its organic tissue quickly, leaving a macerated skeleton of coarse spongin fibres. The consistency is compressible in life, but becomes rigid in preservation. Size of specimens up to 30 x 5 x 4 cm. The skeletal framework of the sponge is made up of coarse thick spongin fibres usually entirely filled with thin, vestigial mycalostyles. Foreign materials including sand grains or algal strands occurs in some of the fibres in some of the specimens but this is uncommon generally. The main fibres, with thickness varying from 100 µm to 1 mm depending on position in the sponge (thicker specimens have basal fibres much thicker than peripheral fibres) may be irregularly anastomosing by connecting thinner fibres (100–250 µm diameter). Surface skeleton is absent. Spicules mycalostyles, microscleres rare or absent. Mycalostyles, typically ‘hollow’, reduced in silica development, almost vestigial, with faintly swollen tyles often grooved or lobed, length variable, 104–280 x 1–7 µm. Anisochelae not found in tropical localities but two anisochelae of 19–23 µm were found in one of Wiedenmayer’s specimens from South East Australia. Sigmas, only rarely observed, absent in tropical localities. Wiedenmayer found 8 sigmas in one of his specimens from South East Australia, thin, widely curved, with apices somewhat incurved, size 42–86 µm.
Distribution. Northern Australia ( Von Lendenfeld 1887; Pulitzer-Finali 1982a), South East Australia ( Wiedenmayer 1989), Northwest Tasmania ( Carpay 1986), on reefs and rocks down to 30 m depth.
Comments. Differences with Red Sea specimens, here assigned to Mycale (Arenochalina) tenuispiculata ( Dendy, 1905) are the color, which is cited as yellowish cream to beige in North Australian, and red in Red Sea specimens. Mycalostyles in North Australian specimens appear distinctly thicker (2–8 µm) than in Red Sea specimens (1–3.5 µm). Of the synonymy designations made by Wiedenmayer (1989), we disagree with M. (Ar.) imperfecta Baer, 1906 , M. (A.) fistulata Hentschel, 1911 , and M. (Ar.) tylostrongyla Pulitzer-Finali, 1982b , as these were reassigned above to the synonymy of Mycale (Arenochalina) imperfecta . This species is purple in color and has abundant microscleres, including rosettes of anisochelae.
Wiedenmayer (1989) also suggested Gelliodes setosa Keller, 1889 was a junior synonym of the present species. However, his reason for suggesting this comes from Van Soest’s (1984) discussion of the affinities of Caribbean Mycale laxissima ( Duchassaing & Michelotti, 1864) , in which he suggested that Indonesian specimen ZMA Por. 01611 showed similarities with M. laxissima . The ZMA specimen was identified by M. Burton as Mycale setosa ( Keller, 1889) , but here it is reassigned to Mycale (Arenochalina) imperfecta (cf. above). The name was also used by Erpenbeck et al. (2016, supplementary data, CO1tree). Burton’s identification (and Van Soest’s remark) does not conform to the type of Gelliodes setosa as described by Keller (1889), a.o. because no anisochelae were mentioned by Keller. As stated above, we examined the type ZMB 270, which conforms to Gelliodes .
Wiedenmayer (1989) did not include South Australian Esperella crassa Dendy, 1896 in his long list of assumed junior synonyms of the present species, perhaps because Dendy mentions the presence of small anisochelae of 16 µm. Other features, including a strong presence of sand and foreign material do suggest it could be a junior synonym. Likewise, Esperella rara Dendy, 1896 with the same sandy skeleton and small anisochelae, but in addition also trichodragmas, appears close if not conspecific with the present species. Both are technically outside our target region, so we refrain from giving summary descriptions. Of the latter species the type material appears to have been lost ( Ayling et al. 1982; Hooper & Wiedenmayer, 1994: 292).
Mycale waitei ( Whitelegge, 1906) (originally as Cladorhiza ) is a likely Mycale (Arenochalina) from New South Wales, but no formal subgenus assignment has so far been made (but see below in Table 9).
Hooper & Wiedenmayer (1994) assigned this species to the genus Naviculina Gray, 1867 . No reasons for this suprising assignment were given. The genus is currently accepted as a subgenus of Mycale species with cleistochelate anisochelae (dubbed ‘naviculichelae’ in this study following Van Soest & Hajdu, 2002) (see below). M. (Ar.) mirabilis does not possess naviculichelae.
Key to the Mycale (Arenochalina) View in CoL species of the region
Remark. We refrain from keying out M. (Ar.) anomala ( Ridley & Dendy, 1886) and M. waitei ( Whitelegge, 1906) as there is doubt of their identity.
1 Growth form a flaring tube or coalescent tubes.............................................................. 2
- Not tubular. Shape is encrusting, lobate or erect club-shaped................................................... 3
2 Color red or pale red; surface with deep valleys and sharp conules................ Mycale (Arenochalina) euplectellioides View in CoL
- Color greyish, whitish or iridescent pale purple; surface with pustular outgrowths, inside more smooth............................................................................................ Mycale (Arenochalina) regularis View in CoL
3 Microscleres obviously present, including rosettes of anischelae, sigmas 40–105 µm ...... Mycale (Arenochalina) imperfecta View in CoL
- Microscleres not common, may be rare or absent............................................................ 4
4 Live color bright red, fibres predominantly filled with mycalostyles, occasionally bryozoans or algae may be enclosed in the fibres, sigmas if present <40 µm ............................................ Mycale (Arenochalina) tenuispiculata View in CoL
- Live color yellowish beige to dirty white, fibres filled with sand grains; sigmas if present> 40 µm ................................................................................................ Mycale (Arenochalina) mirabilis View in CoL
Global diversity and distribution of the subgenus Mycale (Arenochalina) View in CoL
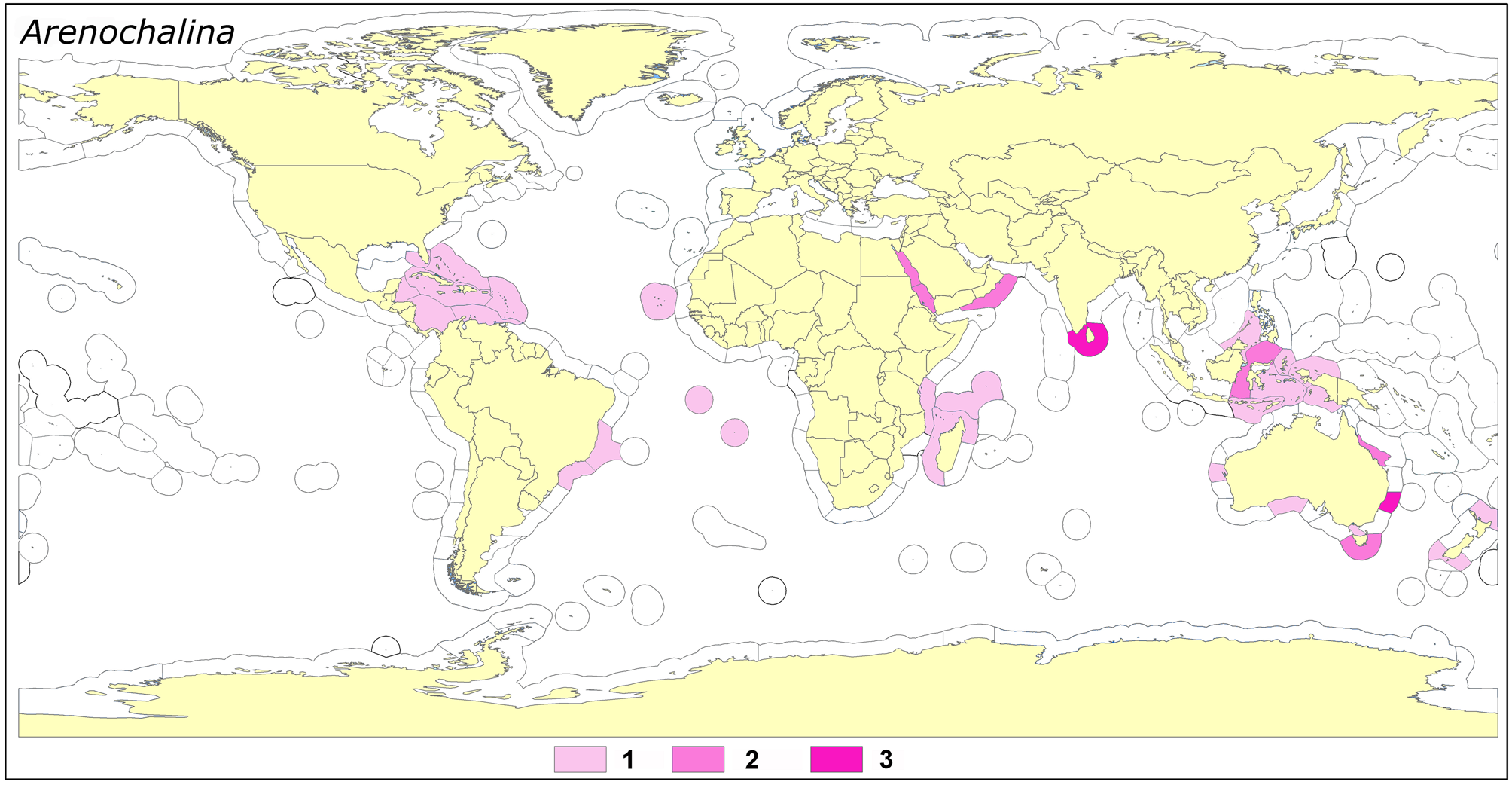
Our estimate for the global diversity of the subgenus is severely uncertain, due to the unsatisfactory state of morphological evidence for species distinction. We believe a rational number of species is approximately 15, but this is not much better than a ‘guess’. One of the vexing problems is the poor knowledge of several South East Australian ‘species’ alleged or suspected to belong to the subgenus. The global distribution of the numbers of species found in Marine Ecoregions (cf. Spalding et al. 2007) is presented in Fig. 32 View FIGURE 32 , showing that the subgenus is absent in polar regions and most temperate waters. Its absence in the Mediterranean indicates it has a circum-tropical distribution with a likely South East Pacific origin.
| ZMA |
Universiteit van Amsterdam, Zoologisch Museum |
| TM |
Teylers Museum, Paleontologische |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
|
SubGenus |
Mycale |
Mycale (Arenochalina) mirabilis ( Von Lendenfeld, 1887 )
| Van, Rob W. M., Aryasari, Ratih & De, Nicole J. 2021 |
Naviculina mirabilis
| Hooper, J. N. A. & Wiedenmayer, F. 1994: 293 |
Mycale (Arenochalina) mirabilis
| Van Soest, R. W. M. & Hajdu, E. 2002: 677 |
| Wiedenmayer, F. 1989: 84 |
| Carpay, M. 1986: 32 |
| Hallmann, E. F. 1912: 252 |
Esperella spongiosa
| Dendy, A. 1896: 16 |
Esperella crassa
| Dendy, A. 1896: 17 |
Arenochalina mirabilis
| Pulitzer-Finali, G. 1982: 100 |
| Whitelegge, T. 1902: 212 |
| Von Lendenfeld, R. 1888: 103 |
| Von Lendenfeld, R. 1887: 821 |