Apistogramma angayuara, Kullander & Ferreira, 2005
|
publication ID |
https://doi.org/ 10.1590/S1679-62252005000300003 |
|
publication LSID |
lsid:zoobank.org:pub:2714C0A6-0D82-40B1-B7B8-714F892F7E3A |
|
persistent identifier |
https://treatment.plazi.org/id/E5937990-D1ED-4670-B585-4A3698FDD18A |
|
taxon LSID |
lsid:zoobank.org:act:E5937990-D1ED-4670-B585-4A3698FDD18A |
|
treatment provided by |
Carolina |
|
scientific name |
Apistogramma angayuara |
| status |
sp. nov. |
Apistogramma angayuara View in CoL , new species Figs. 1-4 View Fig View Fig View Fig View Fig
Holotype. INPA 24058 View Materials . Adult male, 23.7 mm SL. Brazil, Estado do Pará, rio Trombetas right bank, stagnant pool with sand, rock and dry leaves, below cachoeira Vira Mundo. 4 October 1985. E. Ferreira & L. Rapp Py-Daniel.
Paratypes. All from Brazil, Estado do Pará, rio Trombetas drainage. INPA 12645 View Materials , 1 male, 22.6 mm, igarapé at Km 10 on BR-163 , 7 Oct 1985, E. Ferreira & L. Rapp Py-Daniel. INPA 12646 View Materials , 1 juvenile, cachoeira Porteira, quiet water running above rocks , 10 Apr 1985, E. Ferreira & M. Jégu. INPA 12647 View Materials (14) and NRM 37028 (5), 8 males, 18.7-24.7 mm, 6 females 19.9-22.7 mm, and 5 juveniles or sex indeterminable, 12.6-15.6 mm SL, same data as holotype .
Diagnosis. An elongate (body depth 26.4-29.4% of SL) species of the A. pertensis species group, of small size (<30 mm SL), distinguished from other species of the A. pertensis group by fin shape, color pattern and number of infraorbital and dentary lateral line pores as follows: (1) presence of three horizontal rows of prominent dark spots along the abdominal sides vs. absence, except in A. velifera ; (2) both sexes with low dorsal fin and rounded caudal fin vs. dorsal fin lappets prolonged and caudal fin elongately rounded or lanceolate in males of other species, except in A. pulchra , and dorsal fin low in males of A. gephyra ; (3) 2 postlachrymal infraorbital lateralis pores vs. 3 in A. pertensis , A. meinkeni , and A. velifera ; (4) 5 dentary lateralis pores vs. 4 in A. gephyra , A. meinkeni , and A. pulchra ; (5) presence of a caudal spot vs. absence in A. iniridae and A. uaupesi .
Description. Based on holotype with comments on variation in other adults. See Table 1 for summary of morphometric data, and Figs. 1-2 View Fig View Fig for general aspect.
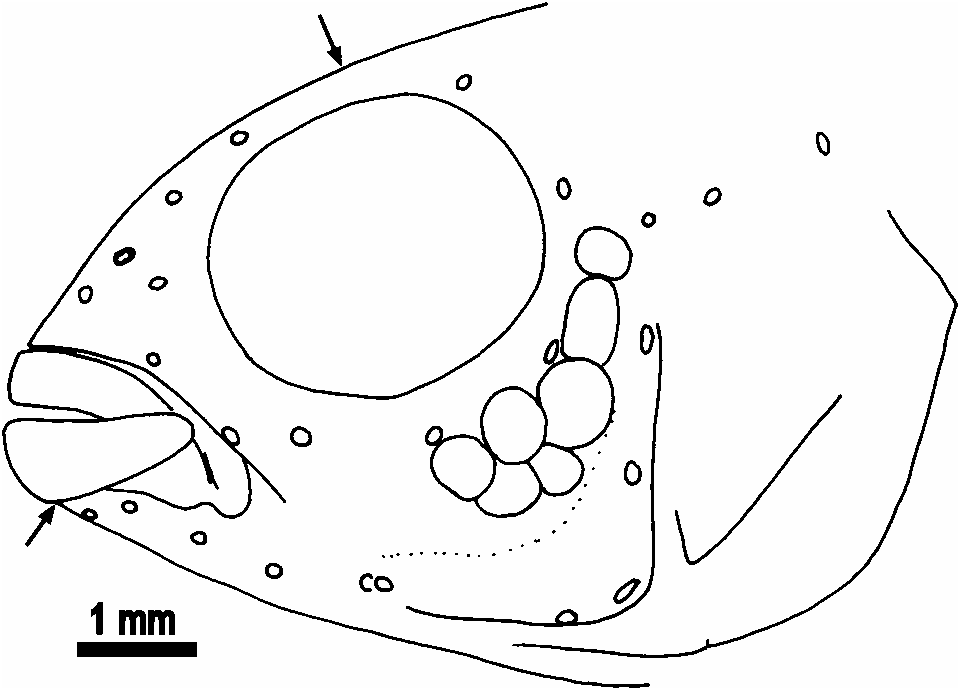
Elongate (body depth 26.4-29.4% of SL), body almost uni- formly deep posterior to orbit. Snout short, with steeply inclined dorsal contour, less strongly sloping ventral contour. Maxilla extending to vertical from slightly posterior to anterior margin of orbit. Eye large, supralateral, its dorsal margin tangented by predorsal contour. Preopercle serrated in 3, supracleithrum in 6 of 10 measured specimens; no posttemporal serrations.
E1 row scales 21 (1), 22 (19). Cheek scaled only posterodorsally, where 1-2 horizontal scale rows developed ( Fig. 3 View Fig ). Predorsal scales 8-10. Prepelvic area naked anterior to tips of cleithra; prepelvic scales 5-7. Scales in transverse row 9, of anterior 1/3 of jaw. Gill rakers externally on first gill arch, one in angle and 1(4), 2(5), 3(1) ceratobranchial; gill rakers on lower pharyngeal tooth-plate 7-9, but difficult to count owing to small size. Lower pharyngeal tooth-plate slightly wider than long (length 85% of width), deeply emarginate posteriorly; 15+15 teeth in posterior row, 6 teeth in median row; most teeth in posteriormost row and larger median teeth of next anterior row tricuspid, shape changing to slender and unicuspid rostrally on bone.
Vertebrae 12+12=24 (19) (count not possible in 2 due to deformities); whereby last abdominal vertebra actually articulating by a short haemal apophysis with first anal fin pterygiophore in 18 specimens. Hypurals 1-5 separate in 19 specimens; hypurals 1+2 and 3+4 fused in one specimen and hypural 3+4 fused in one specimen.
which 7 below upper lateral line. Circumpeduncular scale rows 16. Lateral line scales 13/5 (2), 14/7 (1), 15/5 (1), 15/6 (1), 16/6 (1), 16/7 (2); of which 8-11/0-4 bearing tubes. Scales between upper lateral line and dorsal fin 3 anteriorly, ½ posteriorly. Fins naked except caudal fin, which scaled on basal ¼.
Lateral line canal system on head examined only in alcohol preserved specimens, but obviously a full set of canals and foramina is present, except for 2 instead of 3 postlachrymal pores ( Fig. 3 View Fig ). Dentary with 5 pores; anguloarticular canal present, with anterior and posterior openings and corresponding skin pore, posterior pore separate from anteriormost preopercular pore, however in holotype posterior perforation of skin absent; 6 preopercular pores; coronalis pore present; nasal with pores at each end, posterior obviously shared with frontal canal; frontal with 4 pores; lachrymal bone narrow, with 4 pores; two narrow infraorbital ossicles, anterior with terminal openings, posterior with anterior terminal opening and posterior opening which is slightly anterior to termination of bone (as in Kullander, 1987: fig. 10c).
Dorsal fin low; soft part pointed and extending to about 1/3 of caudal fin in adult males; soft part rounded, shorter, in adult females and young specimens. D. XV.6 (6), XV.7 (13), XVI.5 (1), XVI.6 (1). Soft anal fin pointed, reaching to about 1/ 3 of caudal fin in adult males, rounded and shorter in adult females and young specimens; A. III.5 (2), III.6 (19). Caudal fin rounded, with 3 procurrent and 8 principal rays in each lobe. Pelvic fin pointed, first ray slightly the longer, extending to about vent or first anal fin spine; no sexual dimorphism. Pectoral fin rounded, extending almost to vertical from vent; P. 11 (10).
Jaw teeth caniniform, erect, very slightly curved; 17-21/ 18-23 in upper/lower jaw outer hemiseries. Outer row teeth slightly longer than inner row teeth. Outer row extending along entire jaw margin; one inner row in upper jaw, extending to middle of jaw; one or two inner rows in lower jaw, confined to Color pattern in alcohol. Ground color yellowish white, back with diffuse background pigment pattern with some concentration at scale margins, chest and belly with sparse pigment. No vertical bars. Wide brownish lateral band from gill cleft to end of caudal peduncle, separated from caudal spot by narrow light zone; about one scale deep, margins uneven, chiefly running on scale rows 0 and E1. Dark brown, slightly elongate lateral spot covering E1 row scales 5-7 and half to 2/3 of scales below, and also reflected on adjacent scales above; rarely with slightly lighter marginal zones anteriorly and posteriorly.
Three prominent abdominal stripes composed of a round or deep oval dark brown spot at margin of each scale in rows H1-H3, interconnected horizontally by more or less strongly expressed pigment. Stripes mostly show as rows of dark welldefined spots, but in occasional individuals interspersed pigmentation is so strong that nearly uniform stripes are formed. Dark brown midventral stripe from between pelvic fin bases to base of first anal fin spine. Black spot dorsally and ventrally on axillary side of pectoral fin base.
No supraorbital stripe. Preorbital stripe not well defined in any specimen, but can often be traced in otherwise nearly uniform grayish dorsal snout region. Postorbital stripe brown, from orbit to lateral band. Dark brown suborbital stripe, of about same width as pupil diameter, extending caudoventral from between pores of second infraorbital bone across lower portion of vertical limb of preopercle and onto opercle, preopercle and subopercle in region where those bones meet. Dark brown spot at mandibular tip and adjacent intermandibular region, immediately behind lower lip fold. Snout grayish.
Dorsal fin with dark brown spot at base of each spine and ray, spinous portion smoky with hyaline lappet tips; in several specimens first interradial membrane dark brown, followed by dark brown submarginal stripe. Soft dorsal fin lighter, with 5 or 6 dark stripes across rays. Anal fin with grey lower margin, otherwise brownish, inwardly hyaline and with about 4 rows of dark spots across posterior membranes and rays. Caudal spot midbasal, dark brown, form about square; some pigment above and below spot; on caudal fin distal to caudal
S. O. Kullander & E. J. G. Ferreira 365
spot six prominent vertical stripes, each stripe about as wide as interspace between stripes. Pelvic fins white or hyaline with only a few pigment spots basally.
Females differ from males in less intense fin pigmentation; soft dorsal anal and caudal fins with fewer and only indistinctly expressed vertical stripes or rows of spots. Pelvic fins white or hyaline also in females. Expression of the lateral spot variable between individuals, but a strongly expressed lateral spot may be more frequent in females.
Geographical distribution. Apistogramma angayuara has been collected in the rio Trombetas only in the region of cachoeira Porteira and cachoeira Vira Mundo ( Fig. 4 View Fig ).
Ecology and habitats. The gut content analyses of five specimens (19-24 mm) from rapids showed that aquatic invertebrates were the most important food items consumed by this species. Rhizopods were the dominant item, followed by sponges and cladocerans. Both males and females dissected have well developed gonads.
Collecting at cachoeira Porteira was done in a channel where the Trombetas and Mapuera rivers meet, just above the first waterfall, most of it not deep, but with very fast running water. Lots of aquatic plants ( Podostemonaceae ) grow on the rocks. During the dry season most of the rocks are exposed, and many pools are formed where the fishes are trapped and easily collected with rotenone.
Collecting in the rio Trombetas downstream from the cachoeira Vira Mundo was done on the right bank, just below a channel that connects the river with its upper part during the rainy season. During the dry season this channel dries up, and lots of pools are formed on the banks and over the rocks in which lots of fishes are trapped and easily collected with rotenone.
The igarapé at km 10 on BR-163 is a small clearwater forest stream crossing the road. It is only 1-3 meters wide and shallow, less than one meter deep. On the left side of the road there was a swamp formed by the damming of the stream. Many aquatic plants and algae were present and the water was running fast, but there were many protected places. This stream was destroyed by the logging activities.
Etymology. The species epithet, angayuara , refers to the slender shape of the species and is a Brazilian Lingua Geral adjective meaning skinny or thin (Grenand & Ferreira, 1989). It is used here as a noun in apposition.
Relationships. Apistogramma angayuara conforms to the so-called A. pertensis group distinguished by Kullander (1980: 49) for A. pertensis , widely distributed in the Amazon basin, A. meinkeni , A. uaupesi , and A. gephyra from the rio Negro, A. iniridae from the upper río Orinoco, and A. pulchra from the rio Madeira drainage near Porto Velho. Staeck (2003) added A. inornata and A. velifera from the middle Orinoco drainage in Venezuela. Additional putatively distinct species or color forms are listed in aquarium literature ( Koslowski, 2002; Staeck, 2003), coming mainly from the Orinoco and Negro river basins.
The composition of the group remains provisional in the absence of a phylogenetic analysis, and the original diagnosis of Kullander (1980) has been invalidated by new information and new species described in the meantime. The chief original diagnostic character was the naked anterior chest, which is shared also with A. diplotaenia Kullander , a species of uncertain relationships ( Kullander, 1987). In males of A. pertensis , A. iniridae , A. meinkeni , A. uaupesi , A. inornata , and A. velifera , but not in A. gephyra or A. pulchra , the dorsal fin lappets are prolonged well beyond the spine tips, and at least the posterior lappets are united beyond the spine tips. A similar high dorsal fin with the fin membrane continued beyond spine tips is found in large males of A. borellii (Regan) , a species of uncertain relationships. The caudal fin is rounded to lanceolate in males, but in large males of A. uaupesi the marginal rays are prolonged. There are usually 2 rows of teeth in the jaws, vs. usually 3 in most other species of Apistogramma , but several small species have only 2 rows of teeth, e.g., A. diplotaenia . The absence of a black blotch covering the first 1-3 spines of the dorsal fin is unusual within the genus, and a supraorbital stripe, which is a distinctive mark in most species of Apistogramma , is absent or reduced in size and pigmentation. In other species of Apistogramma , the pigmentation anteriorly on the dorsal fin may vary from deep black as in most of the species to imperceptible in, e.g., A. brevis Kullander , A. personata Kullander , and A. gibbiceps Meinken ( Kullander, 1980) , and the supraorbital stripe is absent or reduced in other elongate species, e.g., A. paucisquamis Kullander & Staeck (1988) . Small specimens show indistinct dark vertical bars, but vertical bars are typically absent in adults, which show a distinct lateral band including a distinct lateral blotch. Most other species of Apistogramma are vertically barred also as adults, and the presence of a lateral spot is variable. Species of the A. pertensis group are comparatively elongate, but shape variation has not been properly analyzed, and several other species, e.g., A. bitaeniata Pellegrin and A. diplotaenia , are also comparatively elongate.
We continue to recognize the A. pertensis group provisionally, including species with the combination of naked anterior chest, absence of a dark spot anteriorly in the dorsal fin, and large males with dorsal fin membranes united beyond spine tips. In large males of A. gephyra and A. pulchra , the dorsal fin lappets remain short, and we agree with Koslowski (2003) that these two species may not belong here. Apistogramma angayuara also has a low dorsal fin, but that may be explained by its small size, since united dorsal fin lappets only occur in specimens over 30 mm SL in the other species of the A. pertensis group. In the absence of a phylogenetic analysis, the A. pertensis group serves as a convenient ad hoc reference for comparing similar-looking species, but it may turn out to be polyphyletic.
Within the A. pertensis group, A. angayuara is distinguished by the prominent abdominal stripes, i.e., three horizontal rows of dark brown spots along the sides of the abdomen, as intense as or more intense than the lateral band. It differs from A. meinkeni , A. velifera , and A. pertensis in the presence of 2 vs. 3 post-lachrymal infraorbital pores, and from A. gephyra , A. meinkeni , and A. pulchra in the possession of 5 vs. 4 dentary pores, sharing the combination of 2 postlachrymal infraorbital pores and 5 dentary pores ( Fig. 3 View Fig ) only with A. iniridae (but see below), A. uaupesi , and A. inornata . Apistogramma iniridae and A. uaupesi are distinguished from other species in the A. pertensis group, including A. angayuara , by the absence of a distinct caudal spot.
The number of openings to the lateralis canal on the head is usually constant in species of Apistogramma . A species may have either 2 or 3 postlachrymal openings (apparently the middle or the posterior opening is absent in the reduced state) and either 4 or 5 dentary openings (apparently the next to posteriormost opening is absent in the reduced state), and abnormal specimens are rare. In addition, the anguloarticular canal is absent in a few small species. Within A. iniridae (N=34), there is a low frequency of three instead of two postlachrymal infraorbital pores, with the two posterior pores closely approximated (3 in 5 specimens, 2 in 28, canal and pores absent in 1), and a bimodal frequency of 4 (18 specimens) or 5 (16 specimens) openings to the dentary canal.
Apistogramma pertensis occurs in the middle and lower rio Negro, lower rio Tefé and rio Preto da Eva, all black-water habitats. Numerous collections from the lower rio Tapajós and from the rio Trombetas upstream to the cachoeira Porteira are tentatively assigned to A. pertensis . Apistogramma pertensis is highly sexually dimorphic, males growing much larger (38.8 mm SL) than females (29.9 mm SL) and developing a high dorsal fin with interradial membranes united beyond the spine tips, and a prolonged pelvic fin ( Kullander, 1980). Apistogramma pertensis also has an inverse sexual dichromatism compared to A. angayuara because the stripes crossing the unpaired fins are more distinct in females than in males. The caudal spot varies in shape between geographical samples of A. pertensis . Usually the caudal spot is large and rounded or vertically ovate, extending across most of the caudal fin base. In some populations, including those from the lower rio Trombetas, however, the spot approaches a rectangular shape and is limited to the central part of the caudal fin base, resembling the spot in A. angayuara .
Although A. pertensis frequently shows concentrations of dark pigment at the scale margins, such a pattern is developed over most of the flanks, and does not develop into prominent stripes or spot rows along the sides of the abdomen as in A. angayuara . Apistogramma inornata may display two indistinct abdominal stripes and A. velifera possesses 3-4 distinct abdominal stripes composed of rows of small dark dots. Other species of the A. pertensis group possess plain abdominal sides with the exception of A. iniridae , which displays a series of large dark blotches immediately below the lateral band.
The absence of pronounced sexual differences in color pattern or fin shape in A. angayuara is remarkable. It is possible that the available males are not fully grown and thus do not display a full typical set of secondary male characters
S. O. Kullander & E. J. G. Ferreira 367
found in other species of the A. pertensis group such as long pelvic fin and high dorsal fin. On the other hand, gonads of several specimens examined are fully ripe and the larger specimens, both males and females, are obviously in breeding condition. With the largest specimen, a male, 24.7 mm SL, A. anguayara is the smallest species so far reported in the genus.
| NRM |
Swedish Museum of Natural History - Zoological Collections |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.