Amphimedon queenslandica, Hooper, John N. A., Van, Rob W. M. & Soest, 2006
|
publication ID |
https://doi.org/ 10.5281/zenodo.273551 |
|
DOI |
https://doi.org/10.5281/zenodo.6256035 |
|
persistent identifier |
https://treatment.plazi.org/id/9A471D23-534D-FFC3-FE85-FD2BFAA0F8CA |
|
treatment provided by |
Plazi |
|
scientific name |
Amphimedon queenslandica |
| status |
sp. nov. |
Amphimedon queenslandica View in CoL sp.nov.
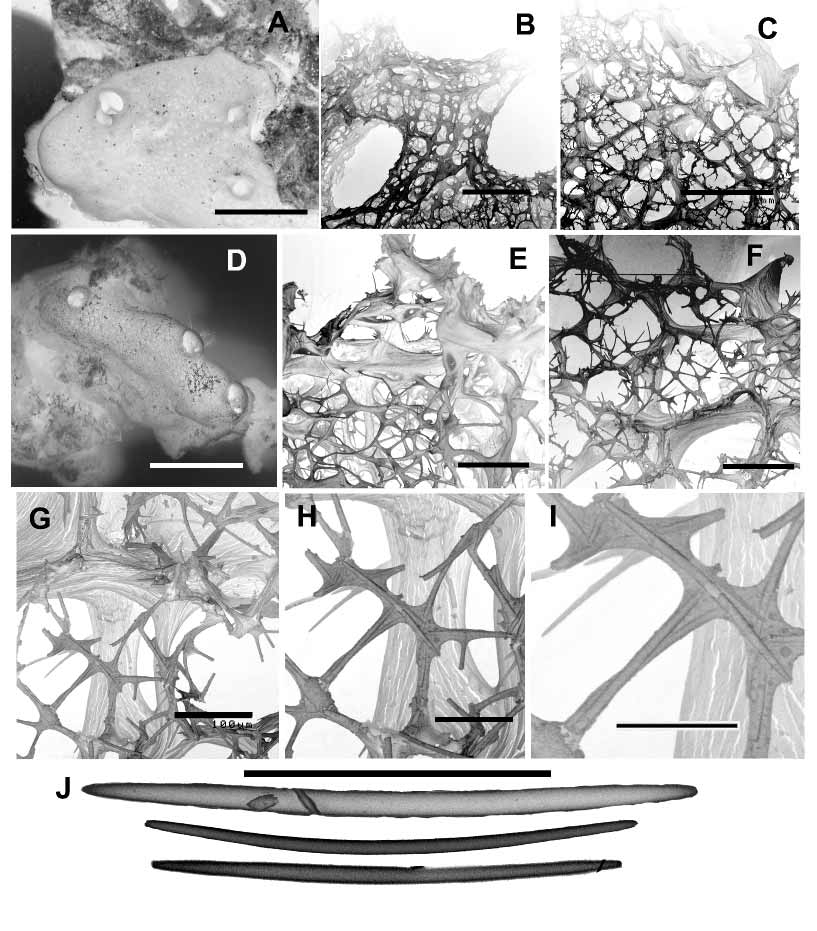
( Fig. 1 View FIGURE 1 )
Reneira sp. [sic], Leys & Degnan, 2001: 324
Material
Holotype: QMG315611 (fragments of holotype: ZMA Por. 19383). Shark Bay, Heron Island, CapricornBunker Group, southern Great Barrier Reef, Queensland, Australia, 23° 27’S, 151° 55’E, intertidal 0.5m depth, coll. Nelson Lauzon & Sally Leys, 10 April 1999, by hand, under coral rubble on reef flat.
Paratypes: QMG322874, QMG322875, QMG322876, ZMAPOR 19384 (fragment QMG322872), ZMAPOR 19385 (fragment QMG322873): Shark Bay, eastern end of sand cay, 50–100m from the shore, Heron Island, CapricornBunker Group, southern Great Barrier Reef, 23° 27’S, 151° 55’E, intertidal <2 m depth, coll. Simon Walker, 8 June 2006, by hand, coral rubble substrate.
Specimens: QMG324489, QMG324490: northern crest, near entrance, One Tree Island, CapricornBunker Group, southern Great Barrier Reef, 23° 29'05"S, 152° 04'24"E, intertidal <50 cm depth, coll. Simon Walker, 6 June 2006, by hand, coral rubble substrate. QMG324491: eastern crest, near “two tree island”, One Tree Island, CapricornBunker Group, southern Great Barrier Reef, 23° 29' 14''S 152° 05' 18'' E, coll. Simon Walker, 6 June 2006, by hand, coral rubble substrate.
Description
Growth form is thickly encrusting over dead coral rubble, forming thin (quoted as> 1mm by Leys & Degnan, 2001) to moderately thick (> 10mm) crusts over the coral, usually also with massive, lobate or digitate bulbs arising from the encrusting base, several centimetres high and in diameter. Sponges are easily removed (cut or torn) from the coral rubble. Oscules are large (10–20 mm diameter) and prominent, situated on the summit of surface bulbs, each oscule surrounded by a slightly elevated membranous lip several millimetres high. Surface is macroscopically smooth, even, translucent, unornamented although microscopically porous and appears ‘slightly fuzzy’ due to it being pitted by numerous very small ostia (<1mm diameter), and with terminal spiculospongin fibres forming minute conulose projections on the surface. The underlying skeletal fibre network is also visible through the partly translucent ectosome. Texture is compressible but firm and very resilient, quickly returning to its initial shape when compressed. Live colouration is greyblue to green, with lighter grey around the rim of the oscule. In ethanol the sponge turns beigegrey to grey, shape is not lost, and it leaks a green pigment when fixed.
The ectosomal skeleton consists of an irregular paratangential reticulation of unipaucispicular spiculofibres, originating from subectosomal endings of larger choanosomal fibres tapering towards the surface producing a slightly microvillose surface. These peripheral fibres are generally more heavily spicular, more densely spaced (‘peripheral condensation’) and have greater amounts of collagen than those within the choanosomal skeleton, including the presence of dark refractive dark granules in the first 200 µm or so of the surface skeleton. These darker denserfibred regions are also repeated several times in the interior skeleton and may be interpreted as evidence of periodic episodes of retarded growth. The choanosomal skeleton is an anisotropic reticulation of moderately well developed thicker primary fibres (30–50 µm diameter, thicker at the fibre nodes), containing loosely packed multispicular or paucispicular tracts of small oxeas, forming oval to elongate meshes (120–300 µm diameter, generally larger in the peripheral skeleton). These larger fibres are interconnected by thinner unispicular, or less commonly paucispicular tracts of oxeas, mostly contained within small fibres but occasionally not covered by collagen, together forming an isodictyal reticulation in between the larger fibre network (with meshes 35–145 µm diameter). Smaller spiculospongin tracts range from 8–16 µm in diameter, thicker at fibre nodes. Collagen is light and homogenous throughout the mesohyl. Leys & Degnan (2001 as a Reniera sp.) report large brood chambers commonly occurring near the base of the sponge, up to 1cm ² in diameter, containing 20–150 embryos at any time in a range of developmental stages, and that the sponges are reproductive throughout the year. Descriptions of the larvae and its behaviour are provided in detail by these authors.
Megascleres are very small and thin oxeas, slightly curved at the centre, with tapering or abruptly rounded points, 55(83)118 µm long, 1.5(2.3)4.5 µm thick. No microscleres were observed although occasional very small sigmas were seen in some sections, considered to be contaminants.
Etymology
The species is named for the political region encompassing the type locality.
Discussion
This present species was originally mentioned as being a Reneira sp. (= Reniera sp.) ( Leys & Degnan, 2001) based on a fragment of a single specimen (QMG315611), but its identification as Amphimedon is confirmed by recollection of several more specimens from the original type locality (Heron Island) and the adjacent One Tree Island in the CapricornBunker Group, southern Great Barrier Reef, and is herein designated a new species. So far the species has not been recorded elsewhere in the Great Barrier Reef, despite extensive recent surveys along its length and breadth. This is perhaps not surprising given its small encrusting growth form and preference for shaded rubble habitat, with most collection effort focussed mainly on the macrobenthic species to date, such that it is possible it has a far more wide distribution than presently known.
Comparison with other Amphimedon species from Australian and adjacent waters. According to Hooper & Wiedenmayer (1994) Chalina polychotoma robusta Carter, 1885 from Port Phillp Heads is an Amphimedon , based on examination of dry type material and a slide. Amphimedon robusta is described as branchingdigitate and orangecoloured ‘when fresh’, thus differing clearly from our new species. The same authors assigned Thalysias massalis Carter, 1886 , also from Port Phillip Heads to Amphimedon based likewise on a dry type specimen and a slide. The growth form is not unlike that of our new species, but the spicules are clearly much larger (155 x 6 µm). The structure of the skeleton is unknown and the Amphimedon status thus uncertain.
PulitzerFinali (1982) described Amphimedon aculeata from Wistari Reef (Capricorn Group), but this is not an Amphimedon as it corresponds to Xestospongia testudinaria ( Lamarck, 1814) (Petrosiidae) . Fromont (1993) described several new species of Amphimedon from the Great Barrier reef area. Amphimedon paraviridis Fromont, 1993 is an olivegreen massively encrusting to lobate sponge with spicules clearly larger (114–204 µm) and thicker (up to 10.5 µm). The surface skeleton is much more regular and there are many single interstitial spicules in the choanosome. Amphimedon lamellata Fromont, 1993 is a distinctly lamellatefolded pinkmauve sponge with spicules also larger (111–156 x 2.5–8.3 µm), and A. sulcata Fromont, 1993 , has a peculiar grooved surface, unlike our new species, and is mauvecoloured. The oxeas are larger (122–153 x 3 –5.3 µm) and there are numerous sigmas. A fourth species, A. terpenensis Fromont, 1993 was assigned to Cymbastela , a genus of Axinellidae (Halichondrida) , by Van Soest et al. (1996) on account of shared skeletal and biochemical attributes, and it is also vastly different from A. queenslandica sp.nov. in its gross morphological features (redbrown lamellate sponge).
Several Papua New Guinean Amphimedon species were described by PulitzerFinali (1996): 129 ( A. fragilis ( Ridley & Dendy, 1886) , A. cristata PulitzerFinali, 1996 , A. conferta PulitzerFinali, 1996 , A. strongylata PulitzerFinali, 1996 , A. rudis Pulitzer Finali, 1996, A. alata PulitzerFinali, 1996 ). The species have been described extremely cursorily, have crude spicule drawings, and lack both habit illustrations and comparative notes. Most of the species have much larger spicules (over 200 µm in length) than our Amphimedon , which makes them suspect wrong assignments. One species, ‘ Amphimedon ’ fragilis , is the type of the genus Dasychalina Ridley & Dendy, 1886 . The only species that has oxeas in the size range of our new species, A. alata , also possesses toxas and is browncoloured.
Two Amphimedon View in CoL species were recorded from the New Caledonian region by DesqueyrouxFaúndez, 1984, viz. Amphimedon conica ( Brøndsted, 1924) and Amphimedon viridis Duchassaing & Michelotti, 1864 View in CoL . The first is an elaborate tubular sponge, originally described from New Zealand ( Brøndsted, 1924 as Pachychalina View in CoL ; Bergquist & Warne, 1980 as Callyspongia View in CoL ). The spicules fall just within the size variation of our new species (105–115 µm but are much thicker: 6–7 µm). However, the colour is described as ‘strawcoloured’ or ‘golden’ and the skeleton (Desqueyroux, 1984: figs 43–45) appears close to that of Callyspongia View in CoL . Amphimedon viridis View in CoL sensu Desqueyroux Faundez, 1984 (not: Duchassaing & Michelotti, 1864) is very probably the same green species as A. paraviridis Fromont, 1993 View in CoL .
Apart from several of the species mentioned above ( A. paraviridis View in CoL , A. conica ), two further Amphimedon View in CoL species have been reported from Indonesia: a recently described Amphimedon denhartogi De Voogd, 2003 View in CoL , which has clearly different shape (lamellate) and spicules (strongyles), and the illknown Phylosiphonia elastica Kieschnick, 1898 (not to be confused with Chalina elastica Kieschnick, 1898 , which is a Callyspongia View in CoL ). Although some clear differences with our new species can be derived from Kieschnick’s (1898, 1900) description, such as the habit (a single tube) and the paucity of spicules in the fibres, there are nevertheless several points in common: it has a wide oscule (11 mm diameter), a shiny surface, and spicules 90–100 µm long. The shape of the spicules, however, appears to differ as Kieschnick describes them as strongylelike with abrupt points. Live colouration is unknown for this species. One related species in Indonesia ( Callyspongia (Euplacella) biru De Voogd (2004) View in CoL , producing interesting chemical compounds, see De Voogd et al. 2005) needs to be mentioned: it shares the blue colour and short spicules (74–89 µm) with our new species. However, the callyspongiid surface skeleton and the ramose shape serve to distinguish the two.
Habitat
At Shark Bay on Heron Island specimens were seen mostly under boulders and crevices, sides and tops of rubble, in sandy patches surrounded by coral rubble, dominated by algae ( Sargassum and Padina ). On the northern crest of One Tree Island specimens were found on the shallow subtidal reef flat and intertidal reef crest, on large pieces of coral rubble in shallow water (<50 cm depth at low tide) or on the hard algal pavement. Habitat is mostly sandy with patches of coral rubble. On the eastern crest of One Tree Island sponges were found on rubble plates on a hard algal pavement that becomes completely drained of water at low tide.
Distribution
So far known only from the CapricornBunker Group, southern Great Barrier Reef.
| ZMA |
Universiteit van Amsterdam, Zoologisch Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Haplosclerina |
|
Family |
|
|
Genus |
Amphimedon queenslandica
| Hooper, John N. A., Van, Rob W. M. & Soest 2006 |
Callyspongia (Euplacella) biru
| De Voogd 2004 |
Amphimedon denhartogi
| De Voogd 2003 |
A. paraviridis
| Fromont 1993 |
Amphimedon conica ( Brøndsted, 1924 )
| Brondsted 1924 |
Phylosiphonia elastica
| Kieschnick 1898 |
Chalina elastica
| Kieschnick 1898 |
Amphimedon viridis
| Duchassaing & Michelotti 1864 |