Adelotremus leptus, Smith-Vaniz, William F. & Rose, Jean Michel, 2012
|
publication ID |
https://doi.org/ 10.5281/zenodo.213220 |
|
DOI |
https://doi.org/10.5281/zenodo.6167734 |
|
persistent identifier |
https://treatment.plazi.org/id/172BE051-FFF2-FFDD-FF14-57A8FEEBEFC6 |
|
treatment provided by |
Plazi |
|
scientific name |
Adelotremus leptus |
| status |
sp. nov. |
Adelotremus leptus View in CoL new species
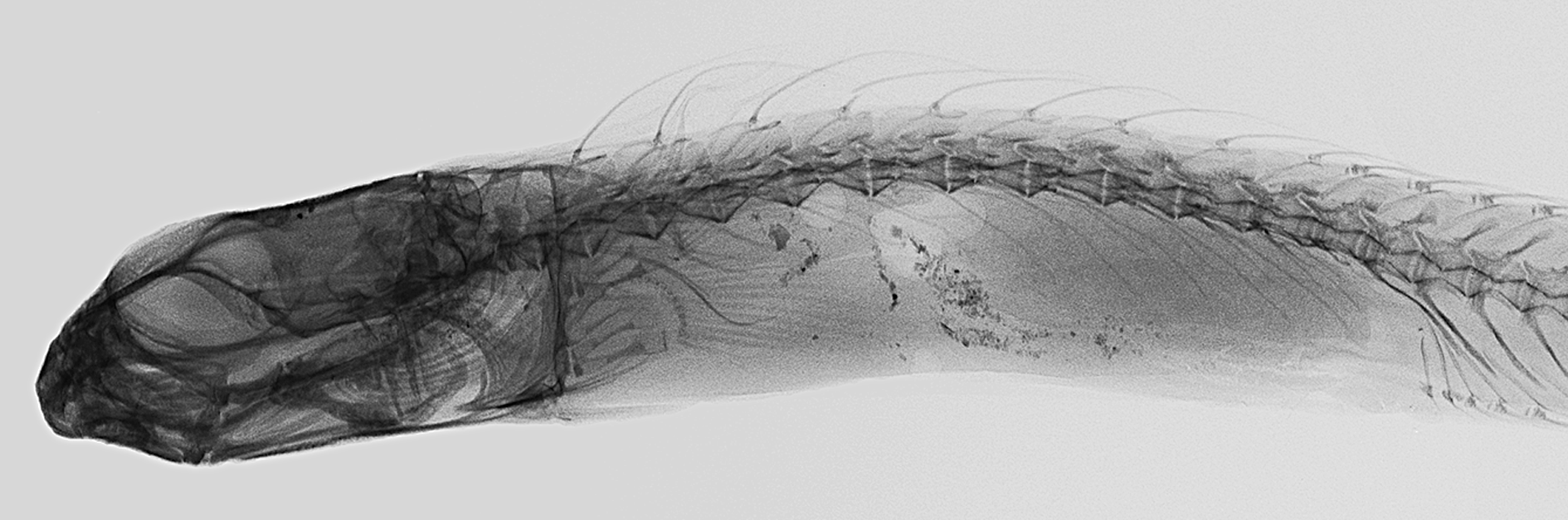
Figures 1‒6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6
Holotype. USNM 402770, 35.4 mm SL, gravid female, Red Sea, Egypt, near Sharm el Sheikh, Naama Bay (Marsa el At), 27°54'38"N, 34°19'44"E, 15 m, Jean Louis Rose, 15 July 2011.
Diagnosis. A species of nemophin with dorsal fin IX, 19; gill opening extending ventrally to opposite 5th or 6th dorsalmost pectoral-fin ray; and total vertebrae 32.
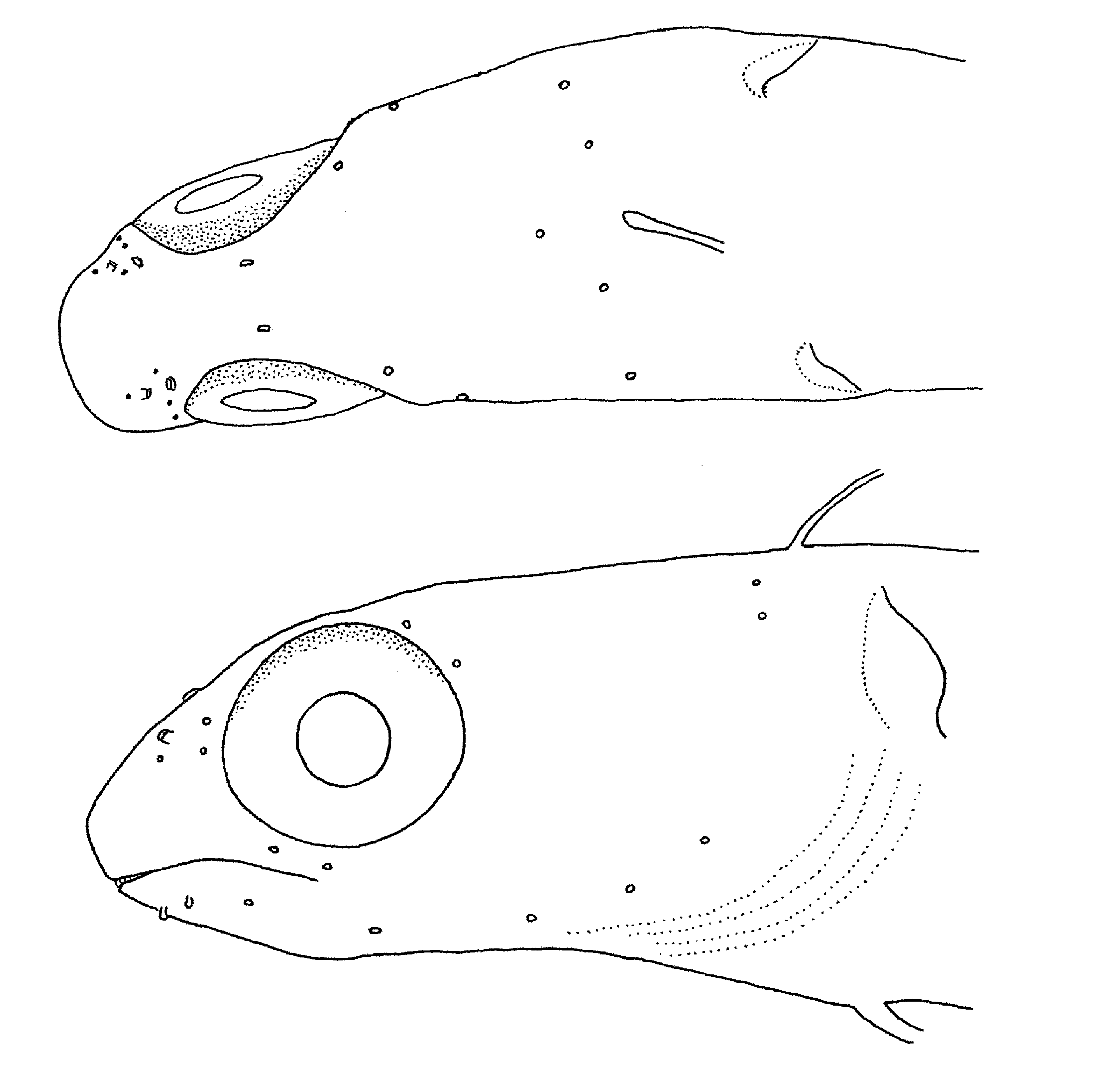
Description. Dorsal fin IX, 19; last ray broadly attached by membrane to caudal peduncle; first spine distinctly shorter than second spine, and spines 2–4 subequal in length. Anal fin II, 19; last ray broadly attached by membrane to caudal peduncle. Caudal fin with 6 procurrent rays (3 dorsal + 3 ventral), 11 segmented rays (6 dorsal + 5 ventral), all rays unbranched; ventral hypural plate fused to urostylar centrum and hypural 5 absent; epurals 1. Pectoral-fin rays 13 (both sides). Pelvic fin I, 3. Vertebrae: precaudal 13 + caudal 19. Dorsal-fin spine pterygiophores broadly contacting robust vertebral neural spines. Posteriormost epineurals and pleural ribs on vertebra 12. Upper and lower jaws each with posterior recurved canines (premaxillary canines much smaller than dentary canines) on each side. Incisor teeth broad based and firmly attached, 26 in upper jaw and 27 in lower jaw. Cranial bones ornamented with numerous small pits. Dentaries connected by tight interdigitating joint at ventral midline. Infraorbital bones 3, including dermophenotic ( Fig. 4 View FIGURE 4 A); second infraorbital slender, elongate and tapering to a point posteriorly; wide gap between second infraorbital and dermosphenotic indicating loss of an infraorbital, which corresponds to absence of infraorbital pores in postorbital region of head. Gill opening with lateral flap only; ventral margin of gill opening opposite level of dorsalmost 5th or 6th pectoral-fin ray. The cephalic sensory pore system ( Fig. 5 View FIGURE 5 ) includes 4 dentary pores, 3 preopercular pores, 2 ventral infraorbital pores and the absence of posterior infraorbital pores, 2 supraorbital pores, 1 pair of interorbital pores, 1 median and 1 lateral supratemporal pore, and 1 posttemporal pore. No lateral line tubes or associated pores. Short palmate cirrus associated with 1st (right side) or 1st and 2nd (left side) dentary sensory canal pores; no cirri associated with preopercular, supraorbital or temporal pores. No supraorbital cirrus. Anterior nostril consisting of very short tube with small flap on posterior margin; posterior nostril with slightly raised rim. Swim bladder not apparent but possibly present; the dead holotype was not fixed in formalin for several hours and in such specimens the delicate swim bladder of nemophins tends to collapse and is then virtually impossible to detect.
Measurements in mm (and as percent SL): Standard length 35.4 mm; head length 8.2 (23.2); eye diameter 2.8 (7.9); preanal length 22.3 (62.8); depth at pelvic-fin origin 4.3 (12.1); depth at anal-fin origin 3.7 (10.5); longest outer (upper) caudal-fin ray 12.6 (35.6); inner caudal-fin ray 6.1 (17.2); pelvic fin 2.4 (6.8); length of first dorsal-fin spine 2.7 (7.6); second spine 4.5 (12.8); third spine 4.9 (13.8) forth spine 4.7 (13.4), spine length measurements only approximate because of small size and curvature of spines.
Fresh coloration ( Fig. 1 View FIGURE 1 ) is essentially the same as the preserved coloration, consisting of brown blotches or small speckles and spots on a tan to white background; iris brown. The most prominent feature of the color pattern is a dark mid-lateral stripe, approximately width of pupil, extending from base of caudal fin through middle of eye on to snout and superimposed by about eight darker blotches with paler interspaces bordered below by indistinct pale spots approximately diameter of pupil; stripe upturned on opercle and forming a point at upper end of gill opening; remainder of body speckled with various shades of brown, except cheeks, opercle and abdomen mostly white; dorsal fin dusky with pale distal margin (wider anteriorly), posteriorly proximal half of fin mostly pale and rays each with several dark spots; anal fin uniformly dusky; caudal fin mostly pale with dusky basicaudal blotch, rays darkly outlined and elongate outer rays brown; pelvic fin white and pectoral fin translucent. Peritoneal lining dark, heavily speckled with large melanophores.
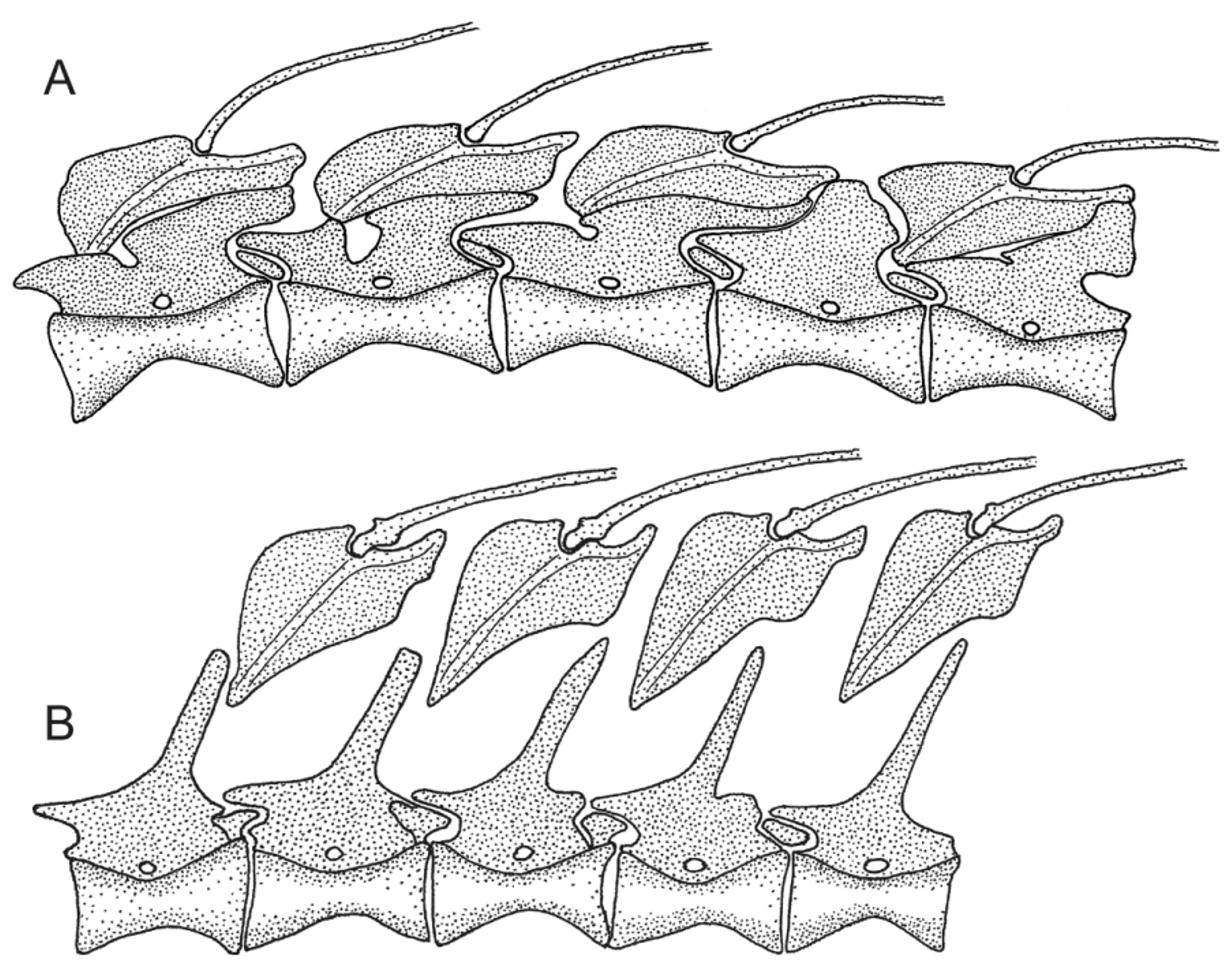
Comparisons. Adelotremus differs from other nemophins (except Xiphasia ) primarily in the nature of its dorsal-fin spine pterygiophores and associated vertebral neural spines, with the pterygiophores broadly contacting robust vertebral neural spines. In the other genera (except Xiphasia ) these pterygiophores either do not contact the usually more slender vertebral neural spines or do so only very slightly. These structural differences are not simply the result of a slender body. The corresponding pterygiophores and neural spines of the very elongate Plagiotremus spilus Gill 1865 or Petroscirtes thepassi Bleeker 1853 ( Fig. 3 View FIGURE 3 B), one of the more slender species of Petroscirtes , do not closely resemble those of Adelotremus . The new genus also differs from other nemophins in having only three infraorbital bones, including the small dermosphenotic, with infraorbital pores absent behind the eye near where the 3rd infraorbital bone would normally be located; the other genera have four infraobitals (see Smith-Vaniz, 1976, Fig. 93), except the subgenera Holomeiacanthus and Allomeiacanthus , which apparently lack a dermosphenotic, and all have a complete series of infraorbital pores.
Adelotremus View in CoL superficially resembles Petroscirtes View in CoL , but, in addition to the characters mentioned above, differs in having the gill opening extending ventrally to opposite the dorsalmost 5th or 6th pectoral-fin rays (versus ventral margin of gill opening above pectoral fin); absence of lateral-line tubes (vs. lateral line extending to near rear of spinous dorsal fin); lateral temporal canal without an associated cirrus (vs. small cirrus present, except in Petroscirtes marginatus Smith-Vaniz 1976 View in CoL and P. pylei Smith-Vaniz 2005 View in CoL ); no orbital cirrus (vs. cirrus typically present in 7 of 11 species); dorsal-fin spines 9 (vs. 10–12 spines, typically 11; in species with 10 spines the frequency of specimens with 10 is usually 4 percent or less); interorbital pores 4 (vs. 2 pores).
The two species of Aspidontus View in CoL agree with Adelotremus View in CoL in having gill openings that extend below the upper margin of the pectoral fin; lateral temporal canal without an associated cirrus; and dorsal-fin spines 9–12 (9 spines present only in A. dussumieri Valenciennes 1836 ). Aspidontus View in CoL differs from Adelotremus View in CoL in having more segmented dorsal- and anal-fin rays, 26–34 and 25–30, respectively (vs. 19 in each fin); pectoral-fin rays 13–15, exceptionally 13 (vs. 13); more total vertebrae 40–48 (vs. 32); interorbital pores 4 (vs. 2 pores); and caudal fin of adults truncate or lanceolate (vs. one very elongate outer dorsal and ventral caudal-fin ray).
Aspidontus View in CoL also is the only nemophin known to have two distinct types of dentary canines (see Smith-Vaniz, 1976: Fig. 29), strongly hooked in prejuveniles with canines of adults only moderately recurved. Although prejuveniles of Adelotremus View in CoL are unknown, it is unlikely they undergo a similar ontogenetic change in dentary canines. The other three genera of nemophins ( Meiacanthus Norman 1943 View in CoL , Plagiotremus Gill 1865 View in CoL and Xiphasia Swainson 1839 View in CoL ) differ from Adelotremus View in CoL in so many other characters (see following key to genera) that a sister relationship with any of them is unlikely.
In the absence of a cleared and stained specimen, a cladistic analysis and hypothesis of the sister-group relationship of Adelotremus View in CoL would be premature. However, if external similarity and overlap in numbers of segmented dorsal- and anal-fin rays and total vertebrae is any indication, the most likely candidate is Petroscirtes View in CoL . We observed a small nubin of bone opposite the prezygapophysis of some precaudal vertebral neural spines ( Fig. 3 View FIGURE 3 A) in Adelotremus View in CoL . Based entirely on examination of radiographs, we also found these structures present, but not consistently, in some species of Petroscirtes View in CoL . The possible phylogenetic significance of these structures would require study of developmental series of cleared and stained specimens and is beyond the scope of this study.
Remarks. The slender body of Adelotremus is well suited for utilization of abandoned calcareous polychaete tubes as shelter ( Fig. 6 View FIGURE 6 ), especially in habitats where other protective cover is scarce. The type locality consisted mostly of sand, broken shells and rubble with occasional seagrass beds present. Some of the more commonly observed fishes in the immediate area and typical of such habitat were Gunnellichthys monostigma Smith 1958 , Ptereleotris heteroptera (Bleeker 1855) , Vanderhorstia ambanoro (Fourmanoir 1957) , V. d e l - agoae (Barnard 1937), V. mertensi Klausewitz 1974 , Fao fo Evermann and Seale 1905 and the rare Enchelyurus petersi (Kossman and Räuber 1877) ; the only other sabertooth blennies seen near the type locality were Aspidontus dussumieri , Petroscirtes mitratus and Xiphasia setifer Swainson 1839 . Rose and Rose (in press) provide a checklist of the fish species known from the Sharm el Sheikh vicinity.
Two other nemophin genera, Aspidontus and Plagiotremus , primarily utilize vacant holes in corals produced by serpulid worms for protective shelter. Aspidontus dussumieri was also observed by the second author and Jean Louis Rose at the type locality occupying polychaete tubes. At least one species of Petroscirtes , P. breviceps (Valenciennes 1836) , has been observed by the first author hiding in a worm tube on a heavily encrusted pier support. The very elongate Xiphasia setifer Swainson 1839 is the only other nemophin known to hide in worm tubes or holes on sandy substrates ( Fig. 7 View FIGURE 7 ). Unlike Adelotremus , the gill opening of Xiphasia is protected by both a lateral and mesial fleshy flap, which probably functions as a one-way valve to prevent sand and fine sediment from entering the gill cavity. Fridman and Masry (1971) observed and collected a 480 mm SL Xiphasia setifer at El-Hamira Bay, Gulf of Elat, positioned vertically in 2.5 m depth that slowly retreated tail first into the sand with only its head protruding from a tube opening. The vertically positioned tube, 2.5 cm in diameter and at least 70 cm in length, was retrieved and illustrated (unnumbered figure). Kuiter and Debelius (1992:244) reported that X. setifer "lives in vertical holes in mud made by other creatures." Their unnumbered color photograph shows a puff of fine sediment produced by a fish backing into a hole.
Adelotremus leptus may prove to be a Red Sea endemic but considering its recent discovery, it could be more widely distributed. Its small size would prevent it from being caught with most bottom trawls, the species' color pattern makes it difficult to visually detect, and the mostly sand and rubble habitat near reefs has not been inadequately sampled.
Etymology. The specific epithet is from the Greek leptos (thin or slender) in reference to the slender body of this species.
| USNM |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Adelotremus leptus
| Smith-Vaniz, William F. & Rose, Jean Michel 2012 |
P. pylei
| Smith-Vaniz 2005 |
Petroscirtes marginatus
| Smith-Vaniz 1976 |
Meiacanthus
| Norman 1943 |
Plagiotremus
| Gill 1865 |
Xiphasia
| Swainson 1839 |
A. dussumieri
| Valenciennes 1836 |