Oreaster reticulatus ( Linnaeus, 1758 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4955.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:E800A72A-C56A-492C-9EE6-FA4F8277DE31 |
|
DOI |
https://doi.org/10.5281/zenodo.4701456 |
|
persistent identifier |
https://treatment.plazi.org/id/FF6987EE-FF9F-FFE2-FF54-416D7E32FE91 |
|
treatment provided by |
Plazi |
|
scientific name |
Oreaster reticulatus ( Linnaeus, 1758 ) |
| status |
|
Oreaster reticulatus ( Linnaeus, 1758) View in CoL View at ENA
Figures 28–29 View FIGURE 28 View FIGURE 29
Asterias reticulata Linnaeus, 1758: 661 View Cited Treatment .
Oreaster reticulatus View in CoL — Tommasi 1970: 10–11, 36, pl. 13, fig. 31; Downey 1973: 60, pl. 24, figs. A–B; Clark & Downey 1992: 293, pl. 72; Hendler et al. 1995: 82, figs. 25–26; Fernandes et al. 2002: 422; Magalhães et al. 2005: 63; Martins & Queiroz 2006: 202–203; Ventura et al. 2007: 238; Manso et al. 2008: 185, fig. 8c–e; Magris & Deìstro 2010: 59, 61; Xavier 2010: 75; Alves & Dias 2010: 157; Benavides-Serrato et al. 2011: 179–180; Miranda et al. 2012: 143–144; Gondim et al. 2014: fig. 8a–g, 12c; Alvarado et al. 2017: S277; Sandino et al. 2017: S294; Souto & Martins 2017: 305, fig. 1C; Agostini & Ozorio 2018: 35; Gurjão & Lotufo 2018: 11; Patrizzi & Dobrovolski 2018: 182; Borrero-Peìrez et al. 2019: 5; Torres & Torres 2019: 413; Cunha et al. 2020: 44 View Cited Treatment , figs. 4E, 8; Magris & Giarrizzo 2020: 3.
Material examined (2 specs, 49–98 mm R). BRAZIL, Bahia (12°52’– 13°02’S; 38°40’– 38°37’W)— Itaparica Island, Ponta de Areia beach, 4.vi.1994, 1 spm R GoogleMaps 49 mm ( UFBA 468 ). Todos os Santos bay , 16 m, 22.v.1997, 1 spm, R 98 mm ( UFBA 367 ) .
Comparative material. BRAZIL. Espírito Santo: Trindade Island , Enseada dos Portugueses (20°29’52.3”S; 29°19’15.6”W), 12.5 m, 23.x.2014, 1 spec, R GoogleMaps 100 mm ( MZUSP 1611 View Materials ); 12.6 m, 6.vii.2015, 1 spec, R 105 mm ( MZUSP 1612 View Materials ) . São Paulo (23º47’– 23º57’S; 45º23’– 46º20’W)—São Sebastião, 1 spec, R GoogleMaps 125 mm ( MZUSP 1956 View Materials ); Santos, 76 m, 1.vi.1999, 2 specs, R 130–140 mm ( MZUSP 1617 View Materials ) .
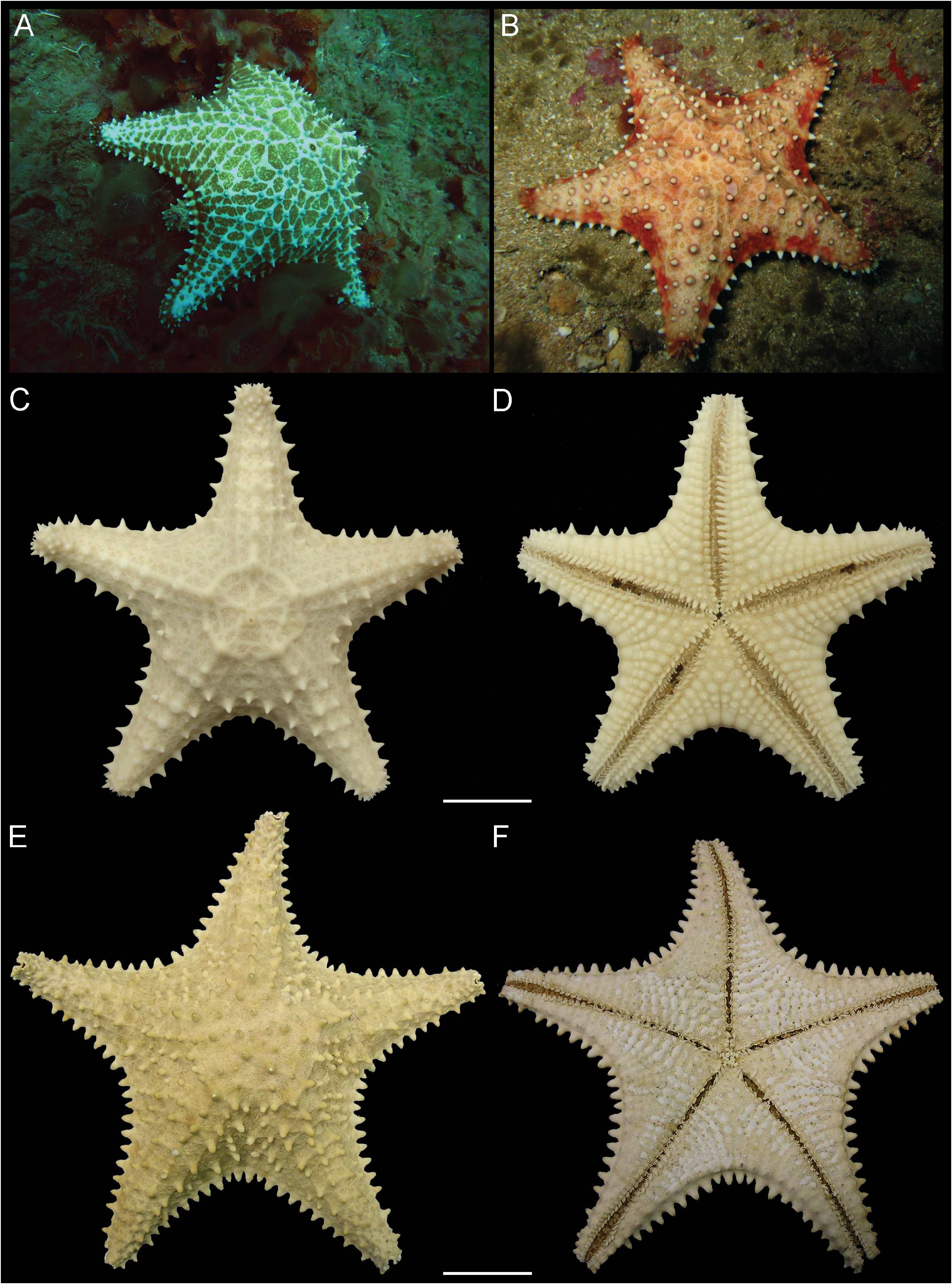
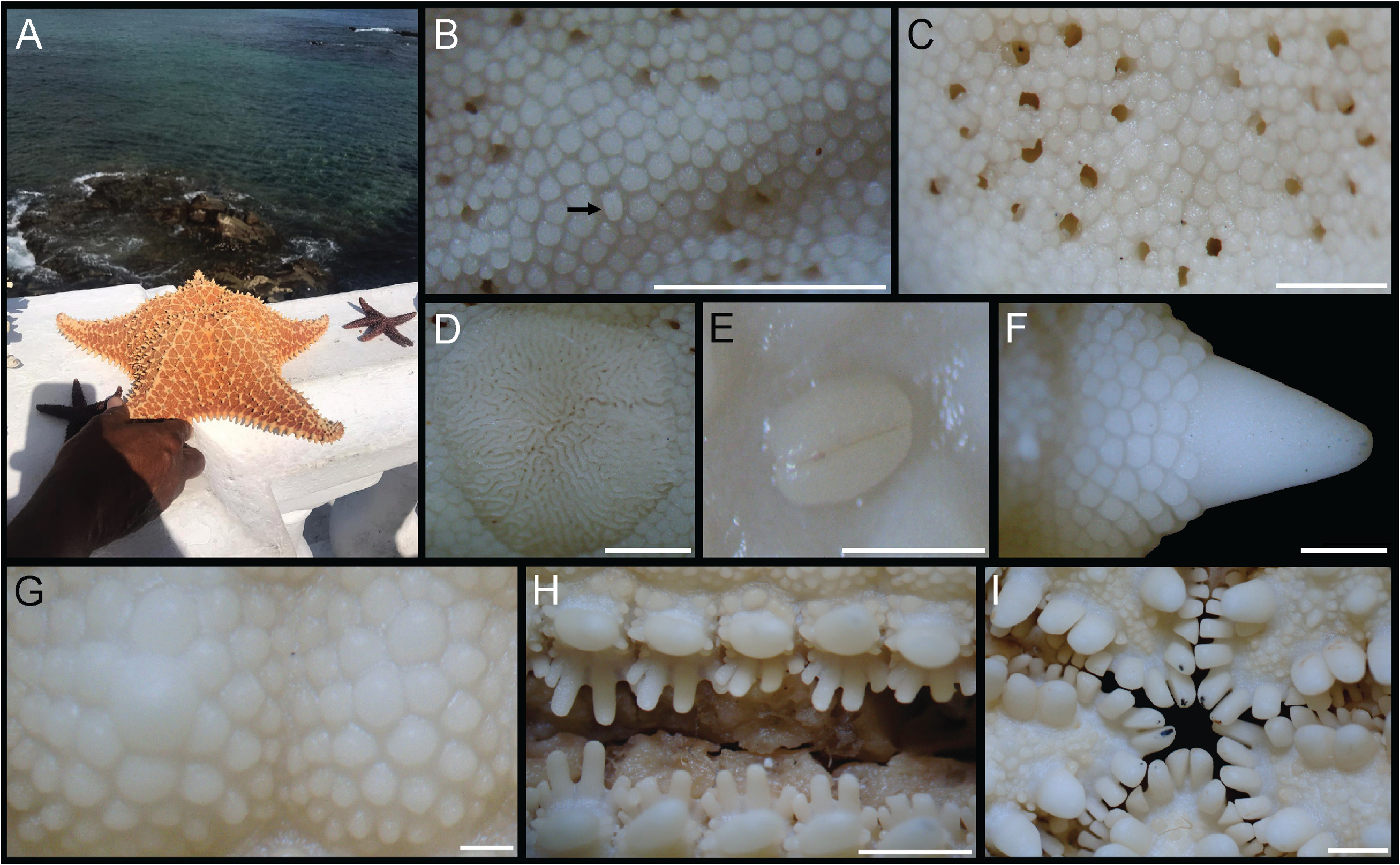
Description (R 98 mm). Body stellate, disc inflated; R/r 1.7 ( Fig. 28A–B View FIGURE 28 ). Five short arms, distally tapering ( Fig. 28E–F View FIGURE 28 ). Abactinal plates reticulate, connected by elongated and narrow secondary plates. Abactinal region with granules, large papular pores, and robust tubercles and spines with blunt tips. Madreporite small, subcircular, covered with shallow gyres. Superomarginal plates large, covered by granules and with a tubercle or a short, robust spine. Inferomarginal plates large, confined to actinal surface, covered by granules and with a tubercle; distal plates with a short and robust spine. Actinal surface with tumid plates covered by granules. Interradial actinal area large. Adambulacral plate with 5–6 unequal spines, spines on extremities smallest. One robust subambulacral spine. Oral plates with five pairs of robust, prismatic spines; inner pair shorter and wider than others. Tube feet in two rows, sucking disc with many perforated plates. Bivalve pedicellariae on both surfaces, more numerous in actinal region, never in alveoli.
Ontogenetic variation (R 49 mm). R/r 1.9 ( Fig. 28C–D View FIGURE 28 ). Some of the abactinal primary plates form an elevated circle from which five rays radiate towards tip of arm ( Fig. 28C View FIGURE 28 ); this pattern was not observed in the larger specimen. Also, the small specimen has five unequal adambulacral spines ( Fig. 29H View FIGURE 29 ), being the proximal spine the smallest, and the third and fourth spines the largest, and the inner pair of spines in the oral plate is longer than the adjacent spines ( Fig. 29I View FIGURE 29 ). Finally, the small specimen has fewer abactinal spines than the largest specimen, which are mostly confined to arms and interradial region (vs. throughout abactinal region), and fewer pedicellariae in the actinal region ( Fig. 29E View FIGURE 29 ).
Coloration. Specimens in vivo have beige to orange abactinal surface, beige to yellowish actinal region and sometimes red spots in the margins. Specimens in ethanol are beige to light brown.
Distribution. U.S.A. (NC, FL), Gulf of Mexico, Mexico, The The Bahamas, Caribbean Sea, Cuba, Belize, Haiti, Dominican Republic, Puerto Rico, Guatemala, Honduras, Nicaragua, Costa Rica, Panama, Colombia, Venezuela, Guyana, Surinam, Canary Islands, Cape Verde ( Verrill 1915; Caso, 1944; Ummels 1963; Walenkamp 1976; Clark & Downey 1992; Hendler et al. 1995; Guzman & Guevara 2002; Entrambasaguas 2008; Hernandéz et al. 2013; Alvarado et al. 2017; Sandino et al. 2017; Borrero-Peìrez et al. 2019; Mah 2020a). BRAZIL: Amapá, Maranhão, Ceará, Paraíba, Pernambuco, Alagoas, Bahia, Trindade Island, Rio de Janeiro, São Paulo, Santa Catarina, Rio Grande do Sul ( Rathbun 1879; Verrill 1915; Tommasi 1958, 1970; Brito 1960, 1962, 1968; Lima-Verde 1969; Walenkamp 1976; Fernandes et al. 2002; Magalhães et al. 2005; Ventura et al. 2007; Magris & Deìstro 2010; Xavier 2010; Miranda et al. 2012; Gondim et al. 2014; Souto & Martins 2017; Agostini & Ozorio 2018; Torres & Torres 2019; Cunha et al. 2020). Depth. 0–76 m ( Clark & Downey 1992; Cunha et al. 2020).
Biological notes. In Bahia, this species is found in protected, shallow waters, often in sandy bottoms with coarse sediment ( Manso et al. 2008). Oreaster reticulatus used to be abundant in shallow waters (intertidal up to 5 m), but locals have reported population declines over the last 40 years; currently, this species is rare and found only in deeper regions. In addition to habitat degradation, especially because of urbanization, this population is affected by human exploitation, as O. reticulatus is commonly sold as souvenirs in tourist shops throughout the country. In November 2008, for example, the Brazilian Institute of the Environment and Renewable Natural Resources (IB- AMA) brought in 15– 20 specimens ( Fig. 28 E–F View FIGURE 28 ) of O. reticulatus that they apprehended, to be identified (unpubl. data). Pinheiro et al. (2018) reported the intense harvesting of O. reticulatus in Espírito Santo, Bahia’s southern neighboring state, with commercial purposes. According to them, specimens are sold by fishermen for US $ 0.50. In Salvador, Bahia, illegally collected specimens have been sold at beaches for US $ 21 (ca. R$120,00) (data from a fisherman interviewed in October 2020; Fig. 29A View FIGURE 29 ).
This species is also used in religious rituals ( Alves & Dias 2010; Souto & Martins 2017), in ornamental aquaria ( Martins et al. 2012) and as medicine to treat asthma, cold and tiredness ( Alves & Rosa 2007; Alves et al. 2009; Alves & Dias 2010; Alves & Alves 2011; Lima 2018). However, the impact of these activities on O. reticulatus populations has not been studied and it is assumed to be low if compared to the commercial harvesting of this species. Franco et al. (2015) reported that extracts of O. reticulatus contain compounds capable of inhibiting the activity of the bacteria Staphylococcus aureus at low concentrations.
Oreaster reticulatus is classified as “Vulnerable” (baseline data indicates that the population size has been reduced by at least 30% as a result of habitat degradation, exploitation and/or introduction of invasive species) by the Ministry of the Environment ( MMA, 2018). This assessment was performed before the devastating oil spill recorded in the Brazilian coast in 2019, which may have a strong impact in the coastal populations of O. reticulatus ( Magris & Giarrizzo 2020) . According to Gurjão & Lotufo (2018), the harvesting of this species in Brazil is currently prohibited. Using models, Patrizzi & Dobrovolski (2018) predicted that the habitable range of O. reticulatus may have a 7–16-fold expansion under higher atmospheric CO 2 concentrations. The effect of this expansion on the local communities is unknown, but it is likely to cause negative trophic impact ( Kordas et al. 2011).
Holotype. NHMD 76271 [previously as ZMUC AST 104 View Materials ] (Tom Schiøtte, per. comm).
Type locality. East Caribbean (as Spanish West Indies) (Tom Schiøtte, per. comm).
Remarks. The specimens examined here as comparative material (R 100–140 mm) have 5–7 adambulacral spines (vs. five in the specimen from Bahia, R 49 mm) and the largest specimens (R 130–140 mm) have two subambulacral spines (vs. one in specimens R 49–105 mm). Gondim et al. (2014) found 5–6 adambulacral spines in specimens ranging from R 51–136 mm, but they did not report if the largest specimens had six spines or if there was no trend in this variation. Also, in specimens with R 100–140 mm, the abactinal and actinal spines are equally developed; however, in the specimen described here, the actinal spines are less developed. Finally, the large specimens do not display the pattern (i.e. elevated circle with rays) observed in the abactinal surface of the small specimen.
H.L. Clark (1933) noticed that small specimens have a deep olive to green abactinal region and as the size increases, the color changes from yellowish– to deep red. Downey (1973) described intraspecific variation (i.e. variation in shape, in degrees of inflation of the disc, and in coloration) in specimens ranging between R 15–91 mm, however, she did not specify ontogenetic changes. We observed that the presence and abundance of abactinal pedicellariae vary between specimens, but this variation does not seem to be related to growth. Also, Downey (1973) noted that O. reticulatus may have 4–7 arms, but both specimens examined here and the comparative material have five arms.
Oreaster reticulatus differs from O. clavatus by having an inflated disc (vs. slightly flattened disc), abactinal plates with tubercles or spines (vs. abactinal plates with granules), and actinal pedicellariae not in alveoli (vs. actinal pedicellariae in alveoli) ( Clark & Downey 1992).
| R |
Departamento de Geologia, Universidad de Chile |
| ZMUC |
Zoological Museum, University of Copenhagen |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Oreaster reticulatus ( Linnaeus, 1758 )
| Cunha, Rosana, Martins, Luciana, Menegola, Carla & Souto, Camilla 2021 |
Oreaster reticulatus
| Cunha, R. & Tavares, M. & Mendonca, J. B. 2020: 44 |
| Magris, R. & Giarrizzo, T. 2020: 3 |
| Torres, V. S. & Torres, F. S. S. 2019: 413 |
| Agostini, V. O. & Ozorio, C. P. 2018: 35 |
| Gurjao, L. M. & Lotufo, T. M. C. 2018: 11 |
| Patrizzi, N. & Dobrovolski, R. 2018: 182 |
| Souto, C. & Martins, L. 2017: 305 |
| Miranda, A. L. S. & Lima, M. L. F. & Sovierzoski, H. H. & Correia, M. D. 2012: 143 |
| Benavides-Serrato, M. & Borrero-Perez, G. & Diaz-Sanchez, C. 2011: 179 |
| Xavier, L. A. R. 2010: 75 |
| Alves, R. N. & Dias, T. L. P. 2010: 157 |
| Manso, C. L. C. & Alves, O. F. S. & Martins, L. R. 2008: 185 |
| Ventura, C. R. R. & Verissimo, I. & Nobre, C. C. & Zama, P. C. 2007: 238 |
| Martins, I. X. & Queiroz, A. C. M. 2006: 202 |
| Magalhaes, W. F. & Martins, L. R. & Alves, O. F. S. 2005: 63 |
| Fernandes, M. L. B. & Tommasi, L. R. & Lima, E. J. B. 2002: 422 |
| Hendler, G. & Muller, J. E. & Pawson, D. L. & Kier, P. M. 1995: 82 |
| Clark, A. M. & Downey, M. E. 1992: 293 |
| Downey, M. E. 1973: 60 |
| Tommasi, L. R. 1970: 10 |