Narcissia trigonaria Sladen, 1889
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4955.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:E800A72A-C56A-492C-9EE6-FA4F8277DE31 |
|
DOI |
https://doi.org/10.5281/zenodo.4701421 |
|
persistent identifier |
https://treatment.plazi.org/id/FF6987EE-FF81-FFFB-FF54-44BD7803FD38 |
|
treatment provided by |
Plazi |
|
scientific name |
Narcissia trigonaria Sladen, 1889 |
| status |
|
Narcissia trigonaria Sladen, 1889 View in CoL View at ENA
Figures 24–25 View FIGURE 24 View FIGURE 25
Narcissia trigonaria Sladen, 1889: 414 View in CoL , pl. 65, figs. 5–8.
Narcissia trigonaria View in CoL — Brito 1960: 5, pl. 1, figs. 4–5; 1962: 3; 1968: 5; Tommasi 1966: 244; Tommasi & Aron 1988: 3; Tommasi et al. 1988: 6; Clark & Downey 1992: 278, fig. 43, pl. 68; Pawson 2007: 54, 58, fig. 3; Magris & Deìstro 2010: 59; Benavides-Serrato et al. 2011: 175; Miranda et al. 2012: 144; Gondim et al. 2014: 35–36 View Cited Treatment , figs. 10f–j; Souto & Martins 2017: 305; Gurjão & Lotufo 2018: 11; Miranda 2018: 14, fig. 10D; Rubio-Polania et al. 2018: 190.
Material examined (10 specs, 7–122 mm R). BRAZIL. Bahia (12°44’– 13°00’S; 38°05’– 38°31’W)—off Bahia, 1 spec, R GoogleMaps 62 mm ( NHM-UK 90.5 .7.641, holotype). Busca Vida beach, 23 m, vii.2005, 1 spec, R 8 mm ( UFBA 1090 ); 23 m, ii.2008, 1 spec, R 16 mm ( UFBA 929 ); 32 m, vii.2008, 1 spec, R 7 mm ( UFBA 1089 ). Busca Vida beach, 1.v.1993, 1 spec, R 95 mm ( UFBA 521 ). Ponta de Areia beach, Itaparica Island , 2000, 1 spec, R 75 mm ( UFBA 469 ). Salvador : 38 m, 2003, 1 spec, R 122 mm ( UFBA 570 ); Barra beach, intertidal, 4.iv.1994, 2 specs, R 70–82 mm ( UFBA 42 ); Porto da Barra beach, x.2008, 1 spec, R 93 mm ( UFBA 962 ) .
Comparative material. Narcissia ahearnae : U.S.A. Florida, Cape Canaveral , 137 m, 25.x.1961, 1 spec, R 90 mm ( NMNH 9736 View Materials , paratype). The The Bahamas. Andros Island, Goat Cay , 52 m, S. Abbott coll., 26.ii.1971, 1 spec, R 120 mm ( NMNH E12440, paratype). Narcissia canariensis : SPAIN. Canary Islands — Tenerife, 1 spec, R 168 mm ( NHMUK 1938.6 About NHMUK .23.1, holotype). Narcissia gracilis : MEXICO. Baja California — La Paz Bay , intertidal, 5.v.1976, 1 spec, R 78 mm ( CASIZ 35025 ); Cabo San Lucas , 6.viii.1932, 1 spec, R 43 mm ( CASIZ 106137 ); 57 m, 1.v.1888, 1 spec, R 50 mm ( NMNH 38317 View Materials , holotype). Narcissia trigonaria: GULF OF MEXICO, 53 m, 7.ii.1885, 1 spec, R 50 mm ( CASIZ 106143 ) ; BRAZIL. São Paulo, 22.ii.1992, 1 spec, R 59 mm ( MZUSP 272 View Materials ); 1 spec, R 58 mm ( MZUSP 315 View Materials ) .
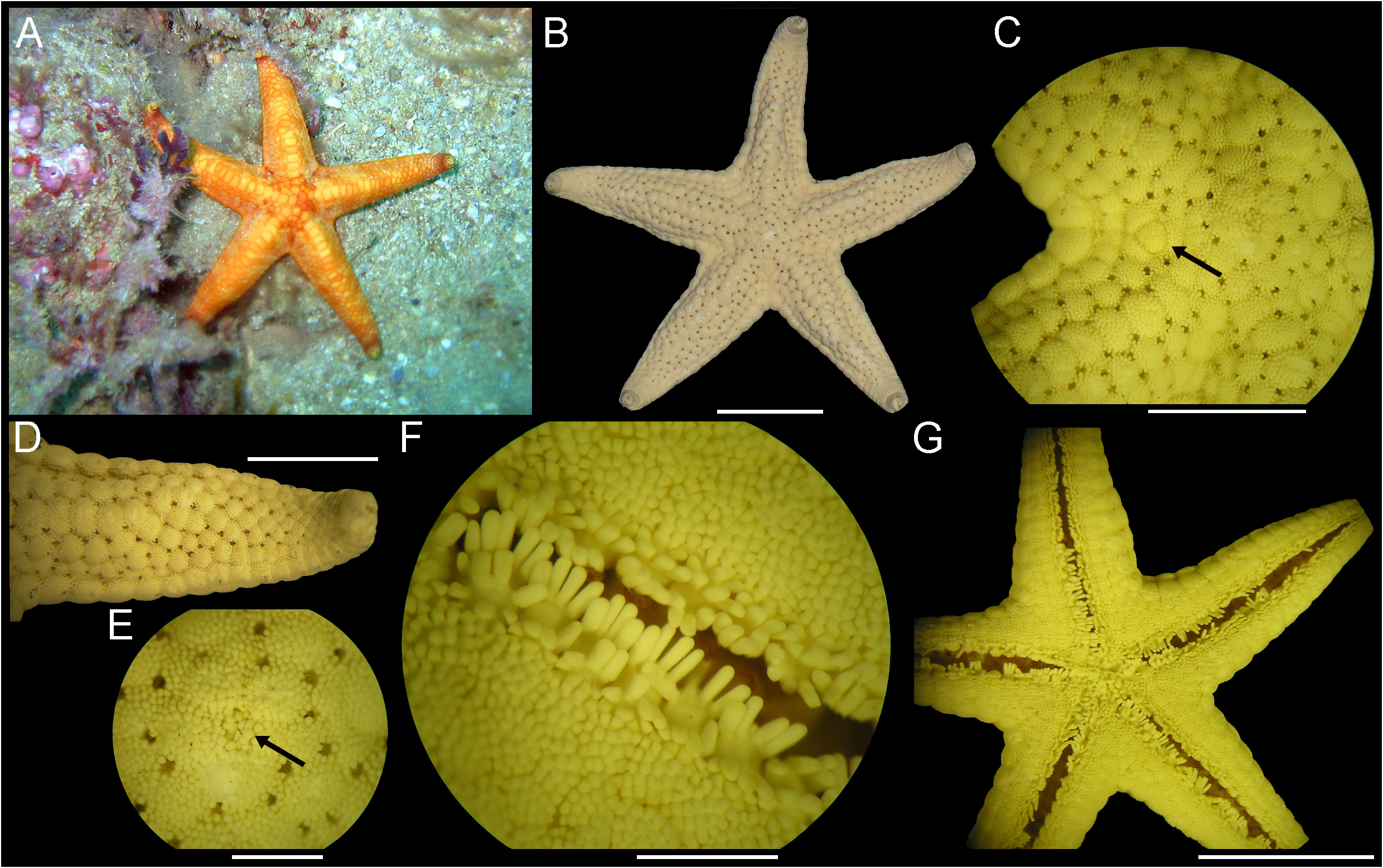
Description (R 70–122 mm). Disc small, pyramidal; average R/r 4.3 ( Fig. 24A View FIGURE 24 ). Arms five, long, distally tapering ( Fig. 24B–C View FIGURE 24 ), cross-section triangular. Carinal ridge undulating in horizontal and vertical planes, from center of disc to middle of arms ( Fig. 24B View FIGURE 24 ). Abactinal plates irregular-shaped, of various sizes ( Fig. 24D View FIGURE 24 ), usually larger along carinal ridge, arranged in irregular series. Abactinal granules round ( Fig. 24E View FIGURE 24 ). Papulae isolated in groups of 2–3 ( Fig. 24E View FIGURE 24 ), surrounded by tightly packed granules; papulae absent in distal region of arm and on actinal region. Madreporite small to medium-sized, varied morphology, in interradius ( Fig. 24F View FIGURE 24 ). Anus evident in center of disc. Superomarginal plates large, mostly confined to lateral surface of arm with only a few proximal plates visible from above. Inferomarginal plates confined to actinal surface. Terminal plates large, squared-shaped, usually bare. Actinal area flat, margins rounded. Actinal plates in four rows, one reaching tip of arm; plates covered by prismatic granules more robust and tightly packed than those of abactinal region ( Fig. 24G View FIGURE 24 ). Four rows of ambulacral spines ( Fig. 24H View FIGURE 24 ). Adambulacral row with 4–5 flattened spines with blunt tip; proximal adambulacral spine shortest, twice as wide as others. Three rows of subambulacral spines. First row with four spines shorter than adambulacral spines; proximal spine smallest and prismatic, other spines similar to those of adambulacral row. Second row with four prismatic spines, proximal spine smallest. Third row with 1–3 prismatic spines, smaller than those of second row and similar to actinal granules. Oral plates with six pairs of large and thick triangular spines, constricted at the base ( Fig. 24I View FIGURE 24 ). Tube feet in two rows, sucking disc present. Pedicellariae absent.
Ontogenetic variation (R 7–62 mm). Average R/r 3.0 ( Fig. 25A View FIGURE 25 ). Compared to the body size, the terminal plate is largest in small specimens, forming the tip of the arm ( Fig. 25B View FIGURE 25 ). Following are additional differences between the smallest (R 7–8 mm), the middle-sized (R 16 mm) and the largest (R 62 mm) specimens examined here: the smallest and the middle-sized (R 7–16 mm) specimens have a flat body, while the largest (R 62 mm) specimen has already a disc and carinal ridge elevated; in R 7–16 mm, all superomarginal plates are visible from above ( Fig. 25D View FIGURE 25 ), but only the proximal plates are seen from above in R 62 mm; R 7–16 mm have single papulae ( Fig. 25C–E View FIGURE 25 ), but R 62 mm has 1–2 papulae per group; in the R 7–8 mm, the carinal ridge is not undulating and all granules are rounded, while in R 16–62 mm, the carinal ridge is slightly undulating ( Fig. 25B View FIGURE 25 ), the abactinal granules are irregular-shaped ( Fig. 25C View FIGURE 25 ) and the actinal granules are prismatic; the actinal plates form one row in R 7–8 mm, two rows in R 16 mm and three rows in R 62 mm; R 7–8 mm have three adambulacral spines (two large and one small), three spines in the first subambulacral row and four spines in the second subambulacral row (third subambulacral row absent or mixed with second row), while R 16 mm has four adambulacral spines (three large and one small), four spines in the first subambulacral row and 5–7 spines in the second and third subambulacral rows ( Fig. 25F View FIGURE 25 ) (separation between rows is not clear), and the ambulacral rows in R 62 mm are fully developed.
Coloration. Specimens in vivo have a cream color with yellow to red-rust large irregular blotches. Specimens in ethanol are white to light brown.
Distribution. U.S.A. (NC, SC, GA, FL, LA, TX), The Bahamas, Gulf of Mexico, Mexico, Honduras, Martinique, Saint Vincent and the Grenadines, Trinidad and Tobago, Panama, Yucatan, Caribbean, Colombia, Venezuela, Guyana, Suriname, French Guiana; Saint Helena Island ( Mortensen 1933; Tommasi 1970; Downey 1973; Walenkamp 1976, 1979; Tommasi & Aron 1988; Clark & Downey 1992; Alvarado et al. 2008; Benavides-Serrato et al. 2011; Rubio-Polania et al. 2018). BRAZIL: Pará, Alagoas, Bahia, Rio de Janeiro and São Paulo ( Sladen 1889; Verrill 1915; Brito 1960, 1962; Tommasi 1970; Tommasi & Aron 1988; Miranda et al. 2012; Gondim et al. 2014; Souto & Martins 2017; Miranda 2018; present work). Depth. 0–210 m ( Pawson 2007b; present work).
Biological notes. In Bahia, N. trigonaria lives in habitats with rocks, corals, calcareous algae and coral rubble, but this species has also been found in muddy substrates in Pará ( Miranda 2018). The Brazilian populations of N. trigonaria are affected by pollutants and illegal collection for the aquarium trade (Brites et al. 2008). This species is classified as “Least Concern” by the Ministry of the Environment ( MMA 2018) and according to Gurjão & Lotufo (2018), its harvesting in Brazil is currently prohibited.
Holotype. NHM-UK 90.5 .7.641.
Type locality. off Bahia, Brazil.
Remarks. The original description of N. trigonaria was based on a R 62 mm specimen and lacked much important information, including on the presence of pedicellariae and the shape of its arm carinal ridge. More than a century after its description, the holotype of N. trigonaria is redescribed for the first time herein. Contrary to the conclusion of Gondim et al. (2014), intraspecific variability observed throughout the geographical distribution (~ 20°N to 24°S) of N. trigonaria suggests that this species is not well-established. For example, although Pawson (2007b) mentioned that pedicellariae in N. trigonaria are abundant, we did not find any pedicellaria on its holotype or in other Brazilian specimens. Specimens from Florida, Gulf of Mexico, Caribbean, Guyana and Suriname, on the other hand, have pedicellariae in abundance ( Clark 1921; Downey 1973; Walenkamp 1976; present work); these have two long and thin valves ending in 2–3 small teeth ( Downey 1973). Also, the literature only describes N. trigo- naria as having a straight carinal ridge, but our observations show that specimens of N. trigonaria from Brazil and Florida have an undulating carinal ridge, while specimens from the Gulf of Mexico have a straight carinal ridge.
N. trigonaria differs from N. canariensis by having four rows of ambulacral spines and pedicellariae lacking or with a long, thin stem (vs. three rows of ambulacral spines and abactinal pedicellariae with a short, thick stem) and from N. gracilis by having abactinal plates irregularly distributed and squared terminal plates (vs. abactinal plates forming regular rows and rounded terminal plates). The intraspecific variation in N. trigonaria led Pawson (2007b) to describe N. ahearnae from specimens previously identified as N. trigonaria from Florida and The The Bahamas. The presence of an undulating carinal ridge, of seven popular pores per square mm, and the rarity or absence of pedicellariae were the traits that he used to distinguish N. ahearnae from N. trigonaria . However, specimens of N. trigonaria from Bahia have an undulating carinal ridge, 3–7 papular pores per square mm and lack pedicellariae. Cunha & Tavares (in review) concluded that N. trigonaria differs from N. ahearnae by having rounded abactinal granules and only the proximal superomarginal plates visible abactinally (vs. pointed abactinal granules and all the superomarginal plates visible abactinally), and noted that, in general, specimens with an undulating carinal ridge have a higher density of pores, possibly because the undulation reduces the distance between the pores. Molecular data of specimens of N. trigonaria and N. ahearnae would help to answer questions about this taxon complex, for example, if N. ahearnae is a valid species and in this case, when these species diverged.
| R |
Departamento de Geologia, Universidad de Chile |
| NMNH |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Narcissia trigonaria Sladen, 1889
| Cunha, Rosana, Martins, Luciana, Menegola, Carla & Souto, Camilla 2021 |
Narcissia trigonaria
| Gurjao, L. M. & Lotufo, T. M. C. 2018: 11 |
| Miranda, A. P. S. 2018: 14 |
| Rubio-Polania, J. C. & Torruco-Gomez, D. & Gonzalez-Solis, A. & Ordaz, J. & Caamal-Jimenez, Y. 2018: 190 |
| Souto, C. & Martins, L. 2017: 305 |
| Gondim, A. & Christoffersen, M. & Dias, T. 2014: 35 |
| Miranda, A. L. S. & Lima, M. L. F. & Sovierzoski, H. H. & Correia, M. D. 2012: 144 |
| Benavides-Serrato, M. & Borrero-Perez, G. & Diaz-Sanchez, C. 2011: 175 |
| Clark, A. M. & Downey, M. E. 1992: 278 |
| Tommasi, L. R. & Aron, M. A. 1988: 3 |
| Tommasi, L. R. 1966: 244 |
| Brito, I. M. 1960: 5 |
Narcissia trigonaria
| Sladen, W. P. 1889: 414 |