Kapsulotaenia nybelini, Chambrier & Brabec & Scholz, 2020
|
publication ID |
https://doi.org/10.11646/zootaxa.4869.4.4 |
|
publication LSID |
lsid:zoobank.org:pub:B88FBB1F-1083-472E-B429-1403BB080E07 |
|
DOI |
https://doi.org/10.5281/zenodo.4562558 |
|
persistent identifier |
https://treatment.plazi.org/id/FE4287AB-FF8E-3C17-FF2C-F923FAE9AB90 |
|
treatment provided by |
Plazi |
|
scientific name |
Kapsulotaenia nybelini |
| status |
sp. nov. |
10. Kapsulotaenia nybelini n. sp.
( Figs. 7 View FIGURE 7 D–F, 9)
Syn. Kapsulotaenia varia of Nybelin (1917), nec K. varia ( Beddard, 1913) Freze, 1965
Type and only known host. Gould’s monitor, Varanus gouldii ( Squamata : Varanidae ).
Site of infection. Intestine.
Type locality. Mt. Glorious , Queensland, Australia .
Additional localities. None.
Type material. Holotype (complete specimen collected on 29.viii.1963; SAM 5714 View Materials — AHC 35934—slide No. 6); 2 paratypes ( SAM 5699 View Materials — AHC 35832—slides Nos. 2 & 3), 2 paratypes ( SAM 5714 View Materials — AHC 35834—slides Nos. 4 & 7).
DNA sequences. Not available.
Material studied. Two slides with several whole-mounted specimens (5 scoleces of K. nybelini and 1 scolex of K. cannoni ), 2 slides with cross sections of scolex and strobila of a specimen from V. gouldii (XP-1105), Mt. Glorious, Queensland, Australia, 26.xi.1962 ( AHC 35832); 7 slides with whole mounts of several specimens (8 scoleces of K. nybelini and 2 scoleces of K. cannoni ), Mt. Glorious, Queensland, Australia, 29.viii.1963 ( AHC 35834).
Etymology. The new species is named after Orvar Nybelin from Sweden, who contributed significantly to the knowledge of tapeworms including those parasitising reptiles in the first half of the 20th century.
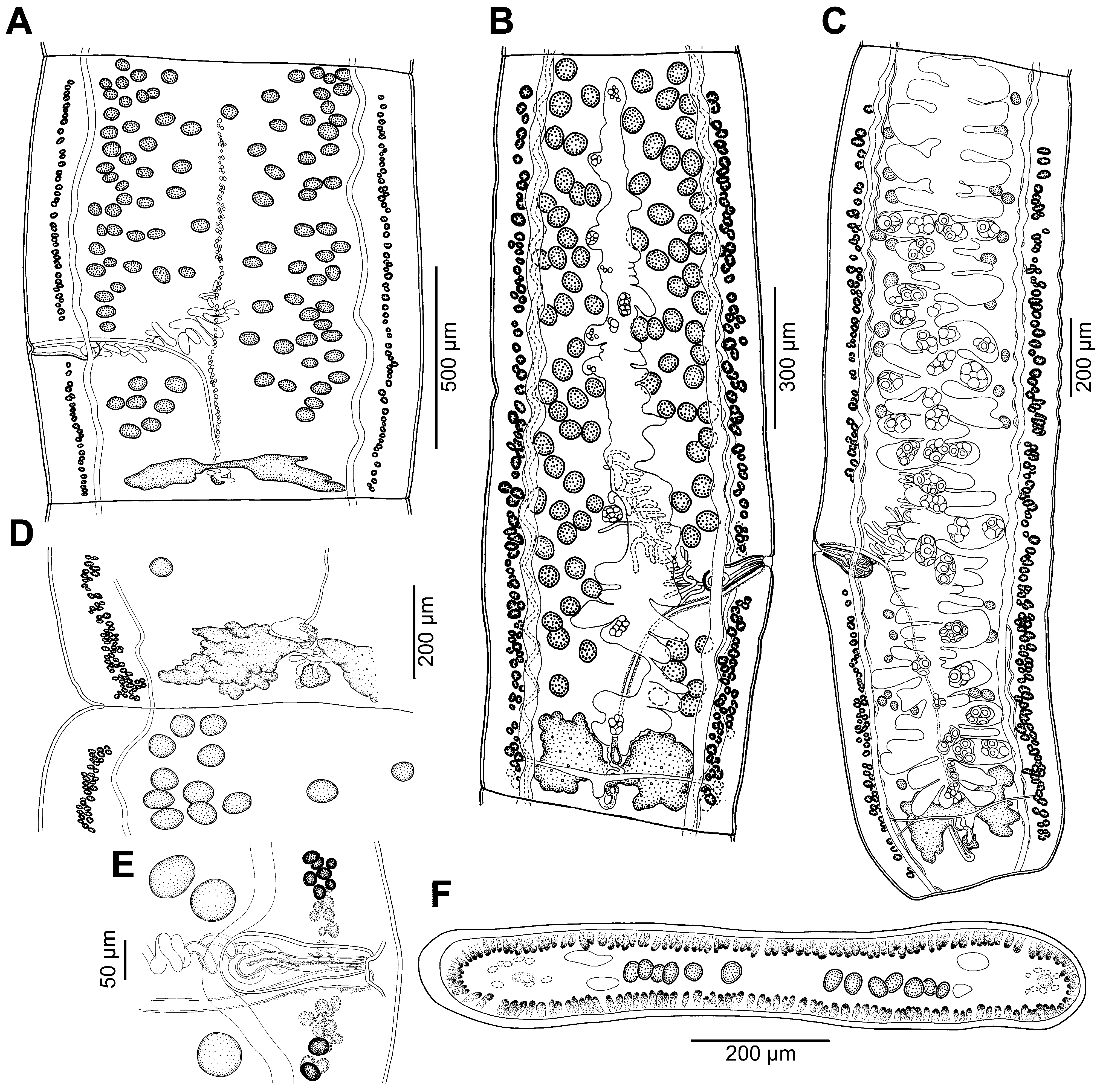
Morphological description (for measurements—see Table 2 View TABLE 2 ). Proteocephalidae , Acanthotaeniinae . Cestodes up to 103 mm in total length; maximum width up to 1,250. Strobila acraspedote, anapolytic. Strobilisation begins about 1.5 mm posterior to scolex, about 39– 81 immature proglottids before appearance of spermatozoa in vas deferens, 1 mature, 7–12 pregravid proglottids, 8 gravid proglottids and 56–100 in total. Immature proglottids wider than long to longer than wide (length: width ratio 0.6–2.10), mature, pregravid and gravid proglottids longer than wide (ratio 1.49–4.25) ( Fig. 9 View FIGURE 9 A–C). Inner longitudinal musculature present at level of proliferation zone, composed of very few muscle fibres; bundles of muscles not observed in mature and pregravid proglottids ( Fig. 9F View FIGURE 9 ). Ventral osmoregulatory canals slightly sinuous, 25–35 in diameter. Dorsal osmoregulatory canals slightly sinuous, 10–15 in diameter ( Fig. 9 View FIGURE 9 A–E). Canals situated between vitelline follicles and testes ( Fig. 9A, C View FIGURE 9 ).
Scolex wider than neck ( Fig. 7 View FIGURE 7 D–F). Suckers uniloculate, spherical, directed laterally to anterolaterally. Rostel-lum dome-shaped, containing large apical organ, posteriorly surrounded by numerous circular muscular fibres ( Fig. 7D, E View FIGURE 7 ). Retractor muscles connecting rostellum with neck present.
Testes medullary, in 1 layer, rounded to elongate, in 2 wide longitudinal bands on both sides of proglottids, with poral band separated by terminal genitalia into preporal and postporal groups ( Fig. 9A, B View FIGURE 9 ); testes and ovary degenerate in gravid proglottids. Cirrus-sac elongate, thick-walled ( Fig. 9E View FIGURE 9 ). Cirrus short, covered by spinitriches, its length representing up to 40% of cirrus-sac length ( Fig. 9E View FIGURE 9 ). Internal sperm duct strongly coiled. External sperm duct ( vas deferens) strongly coiled, directed anteriorly, situated between proximal part of cirrus-sac and midline of proglottid, often crossing it ( Fig. 9A, B View FIGURE 9 ). Genital atrium shallow ( Fig. 9E View FIGURE 9 ); genital pores alternating irregularly, situated in posterior part of proglottid ( Fig. 9 View FIGURE 9 A–C).
Ovary bilobed, butterfly-shaped ( Fig. 9 View FIGURE 9 A–D). Mehlis’ gland small ( Fig. 9D View FIGURE 9 ). Vaginal canal straight in proximal part, enlarged to form small seminal receptacle anterior to ovarian isthmus ( Fig. 9B View FIGURE 9 ). Terminal (distal) part of vaginal canal ( pars copulatrix vaginae) surrounded by few chromophilic cells ( Fig. 9E View FIGURE 9 ). Vagina anterior (28%) or posterior (72%, n = 47) to cirrus-sac ( Fig. 9 View FIGURE 9 A–C, E). Vitelline follicles arranged in 2 longitudinal bands near lateral margins of proglottids (one to three rows of follicles), interrupted at level of cirrus-sac and vagina ventrally ( Fig. 9 View FIGURE 9 A–C, E).
Primordium of uterine stem ventral, present in immature proglottids ( Fig. 9A View FIGURE 9 ). Development of uterus of type 1 according to de Chambrier et al. (2004, 2015). In pregravid proglottids, uterus occupies up to 89% of proglottid length, with lateral diverticula on each side, not overlapping ovary. In gravid proglottids, uterus occupies up to 92% of proglottid length, with 30–36 lateral diverticula ( Fig. 9C View FIGURE 9 ). Uteroduct enters uterus anterior to ovarian isthmus. Intrauterine eggs spherical, grouped in clusters of eggs (number of eggs not known). Unripe embryophore measured in whole mount slides 25–33 in diameter.
Differential diagnosis. The new species is characterized by a large, quadrangular scolex (other species of Kapsulotaenia from Gould’s monitor possess a much smaller scolex—see above). In the width of the scolex, K. nybelini resembles K. sangroundi and K. pythonis , but differs from K. sandgroundi by the position of the vagina to the cirrussac (anterior 28% versus posterior), by the shape of proglottids (longer and narrower versus shorter and larger) and by a smaller scolex (470–645 μm versus 640–900 μm). From K. pythonis , it differs by the number of eggs in clusters (6–8 versus 19–22) and by the size of the scolex (470–645 μm versus 890 μm). Kapsulotaenia nybelini differs from K. chisholmae by a smaller cirrus-sac ratio (17% in average versus 24%) and by the position of the vagina in relation to the cirrus-sac (mainly anterior in K. chisholmae versus mainly posterior in K. nybelini ). The new species can be distinguished from K. tidswelli by slightly narrower ovary (relative width of the ovary 51–58% versus 63–72% in the latter taxon), and by longer bands of vitelline follicles (relative length of bands of vitelline follicles 90–97% versus 84–90%). From K. beveridgei , K. nybelini can be distinguished by a distinct width of the scolex (470–645 μm versus 335–475 μm), and by a different number of uterine lateral diverticula (30–36 versus 17–20). From K. frezei , K. nybelini differs by the shape of the egg clusters (oval versus banana-shaped). From K. varia , it differs by a smaller relative size of the ovary (2.4–4.2% versus 5.2%).
Remarks. The new species resembles in its morphology tapeworms found by Mjöberg in V. gouldii from Mount Tambourine (= Tamborine Mountain), South Queensland, Australia. Nybelin (1917) identified these tapeworms as Acanthotaenia varia (= Kapsulotaenia varia ) based on their presumed conspecificity with Beddard’s (1913) species.
Nybelin (1917) provided illustrations of the scolex (his fig. 1), section through the anterior part of the scolex (fig. 2) and mature proglottid (fig. 3). The longest specimen had a total length of 230 mm; maximum width was 1.5–2 mm. The scolex was reported as only weakly separated from the remaining body, 400–420 µm wide, with spherical suckers 200–220 µm in diameter. The apical organ measured 120 × 130 µm and the cirrus-sac 150–180 × 75–96 µm.
Nybelin (1917) pointed out a high variation in the number of testes (96–158, but usually about 120) and their distribution (sometimes forming two medially well-separated longitudinal fields, sometimes confluent). Nybelin (1917) also observed variation in the extent of vas deferens, the loops of which reach over the mid-line of proglottids to the aporal half, whereas in others not, and relative position of the gonopore (in the posterior fifth to third of the proglottid length).
Only museum specimens of the new species were available and we were unable to obtain the type material of Nybelin (1917). Therefore, it was uncertain whether Nybelin’s material is conspecific with the new species. Furthermore, no molecular data are available to assess phylogenetic relationships of this parasite of Gould’s monitor.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |