PROTOGRYLLIDAE, AND
|
publication ID |
https://doi.org/ 10.5252/zoosystema2023v45a24 |
|
publication LSID |
lsid:zoobank.org:pub:8D29DD39-7ADC-4B36-8E95-3A3F930301D6 |
|
DOI |
https://doi.org/10.5281/zenodo.10392058 |
|
persistent identifier |
https://treatment.plazi.org/id/FB7687BD-FFD4-C73E-74CC-FD7658A724A7 |
|
treatment provided by |
Plazi |
|
scientific name |
PROTOGRYLLIDAE |
| status |
|
FOREWING VENATION PATTERN IN FOSSIL † PROTOGRYLLIDAE View in CoL AND † BAISSOGRYLLIDAE
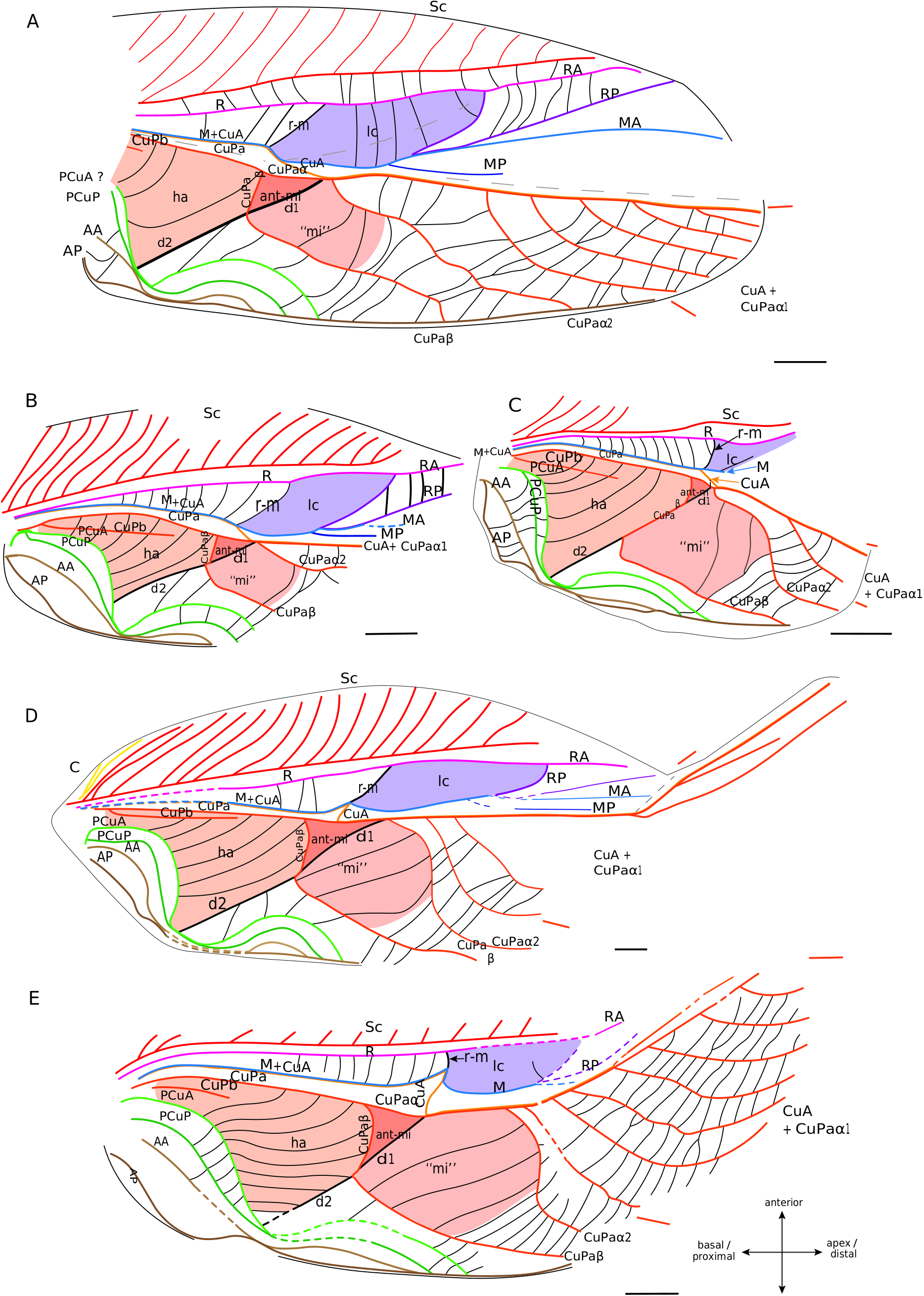
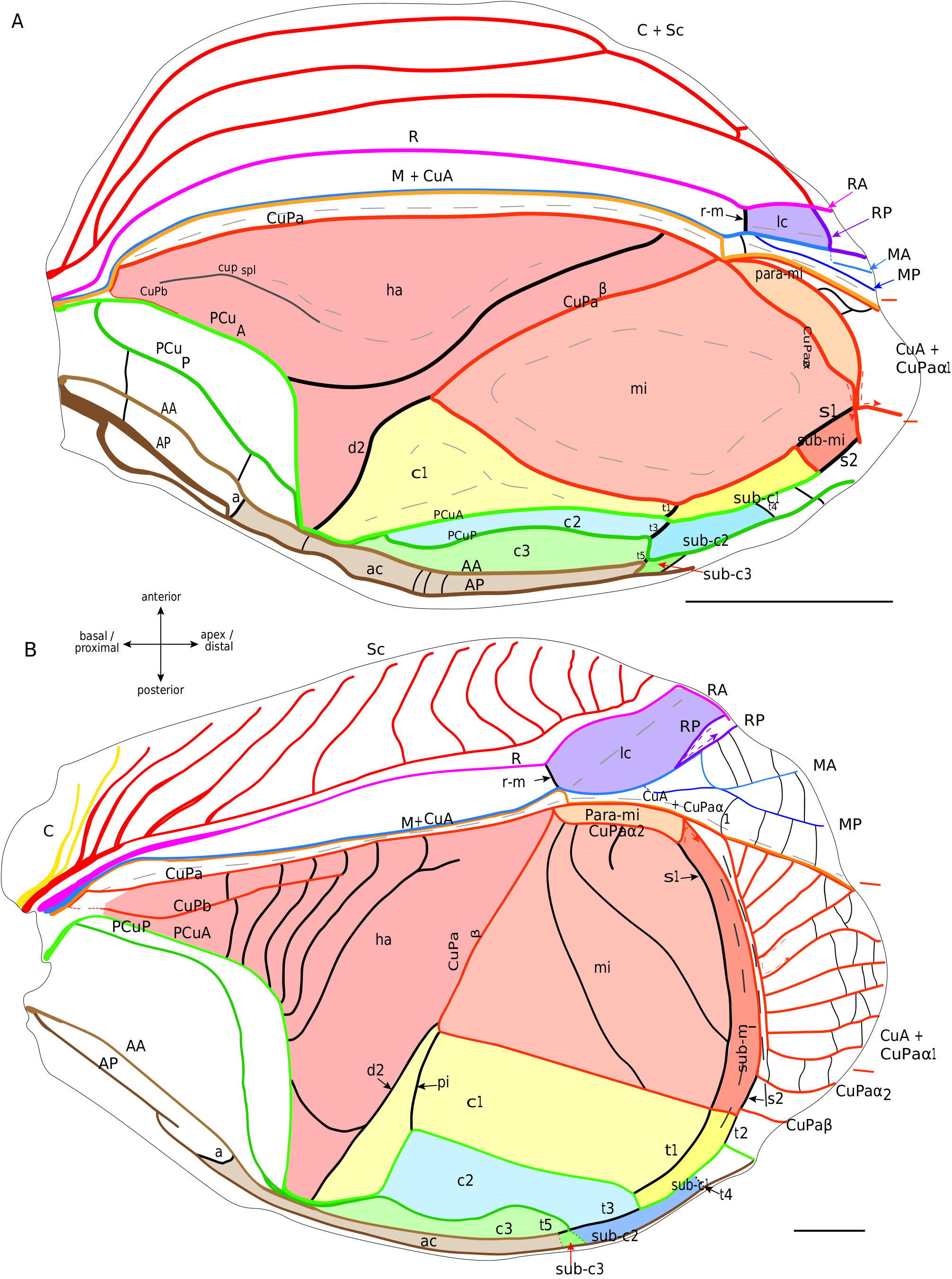
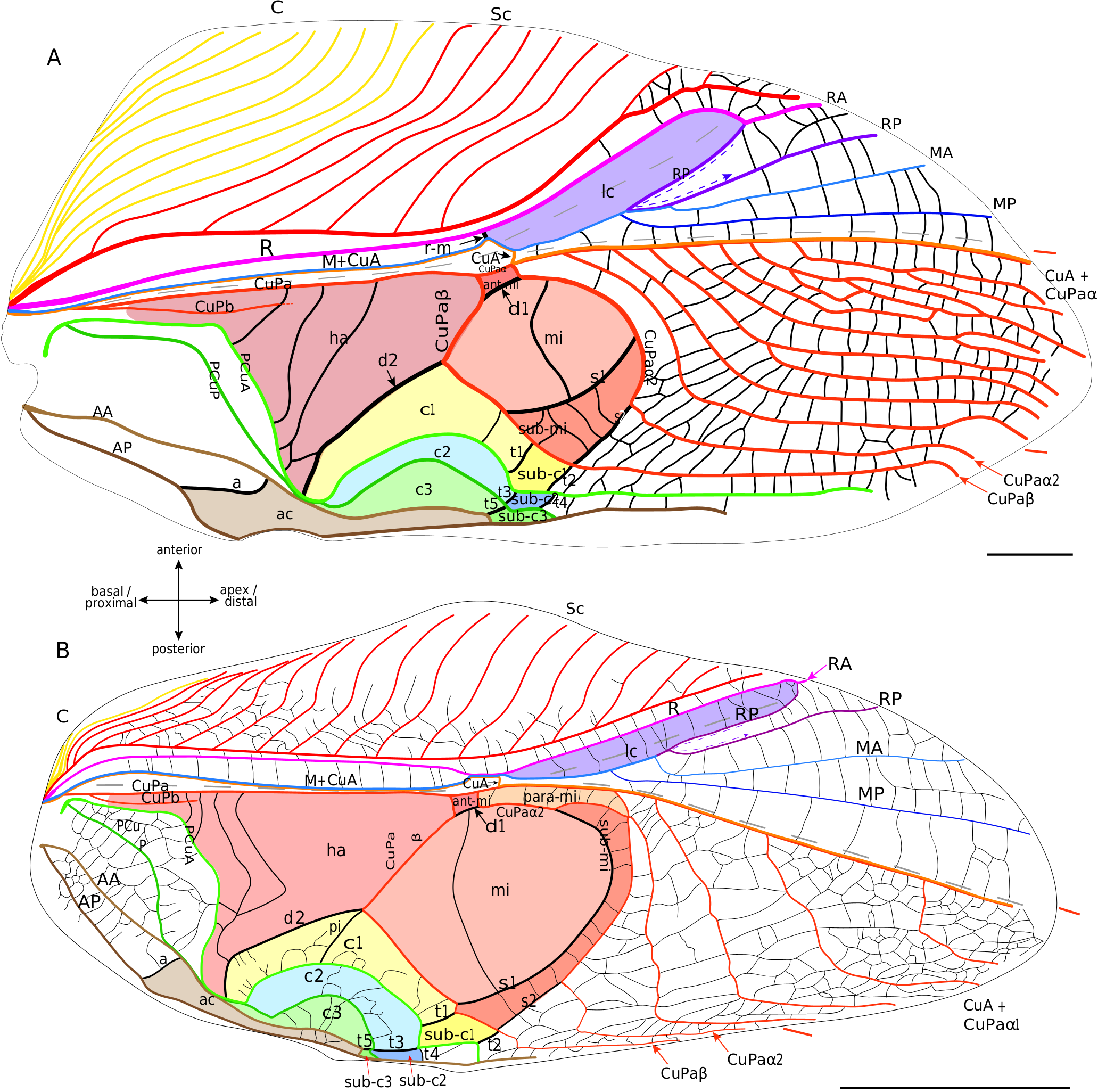
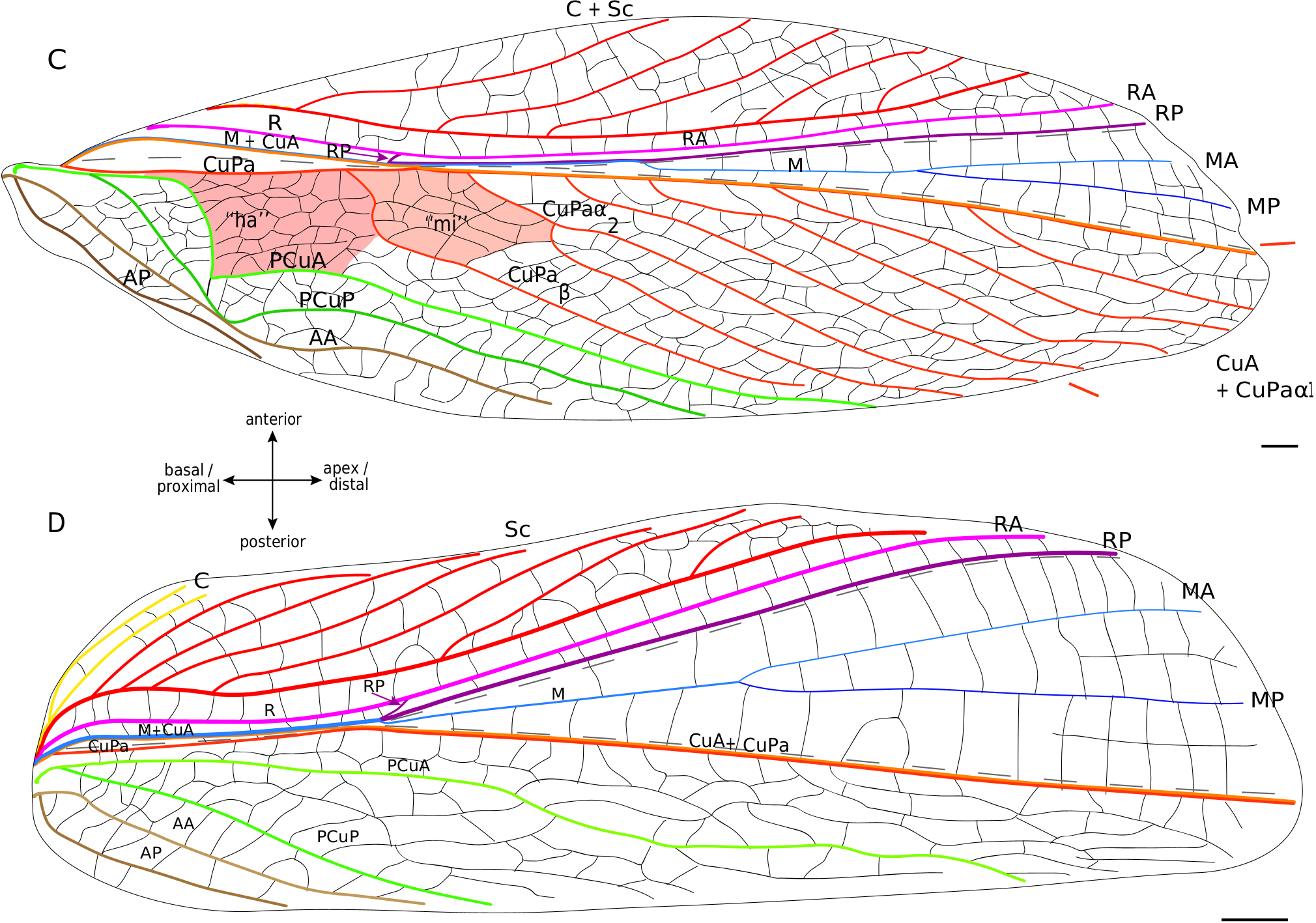
As currently defined, the † Protogryllidae and † Baissogryllidae show a similar and relatively stable venation patterns ( Pérez de la Fuente et al. 2012; Wang et al. 2019). The general organisation of their forewings is almost similar to that of modern crickets, with a lateral field and a dorsal field, a median fold, and a fan between R and CuA (possibly corresponding to a flexible zone, as in mole crickets).
Lateral field. In these fossils, the location of the putative C is often not preserved. The most anterior visible vein is Sc, which has the same pectinate branching as in modern Grylloidea ( Fig. 6 View FIG ). Posteriorly, R is present and strongly convex. Then, the M is fused basally with CuA. A lanceolate cell ( Fig. 6 View FIG ) is present between R and M: it is basally closed by a short transverse vein (r-m) located at the level of the divergence of M with CuA, and distally closed by a curved RP that partially merges with MA (as in modern Grylloidea , see above). Contrary to the latter, some † Protogryllidae have several secondary transverse veins in the lanceolate cell, as for example † Angarogryllus angaricus ( Sharov, 1968) ( Fig. 6A View FIG ). In others, like † Falsispeculum karatavicum ( Sharov, 1968) ( Fig. 6B View FIG ), veins between R and M+CuA at the base of the lanceolate cell are very similar and none is stronger than the others, complicating the identification of r-m among the crossveins. This crossvein r-m can either have an oblique direction towards wing base (obliquely inverted) between R and M ( Fig. 6A, B, D View FIG ), or be rather transverse ( Fig. 6E, C View FIG ). Some † Baissogryllidae (e.g., † Anglogryllus lyristes Gorochov, Jarzembowski & Coram, 2006 , Fig. 6E View FIG ) have a short r-m making a ‘constriction’ of the base of the lanceolate cell ( Fig. 6E View FIG ), a situation similar to that of the modern Grylloidea ( Figs 3 View FIG ; 4 View FIG ; 5A View FIG ).
R divides distally into a convex anterior branch RA and a rather concave posterior branch RP, well visible in † Angarogryllus angaricus ( Appendix 3: Fig. S5A View FIG ). RP is strongly curved basally and partially fuses with MA. The latter, also well visible in † Angarogryllus angaricus , is clearly convex. The fan is often very poorly preserved in fossils, especially for the MP branch. However, applying a conservative approach, we consider that M divides into two branches MA and MP (notably slightly visible in the unidentified † Baissogryllidae no. CCNH-293, Fig. 6D View FIG ). These two branches are relatively thin and present in the fan (a situation similar in the modern Grylloidea ).
Dorsal field. The clearly convex CuA diverges from M and joins CuPa crossing the median fold (well visible in † Angarogryllus angaricus , Appendix 3: Fig. S5A View FIG ). In the † Protogryllidae and some † Baissogryllidae , the CuA is longer and oblique ( Fig. 6A, B, C View FIG ), whereas in other † Baissogryllidae , it is transverse between the two fields ( Fig. 6D,E View FIG ). CuA merges with CuPaα, after its bifurcation with CuPaβ and before the bifurcation between CuPaα1 and CuPaα2. Between CuPaα and CuPaβ, there is a strong crossvein, which corresponds to d1 ( Fig. 6 View FIG ). Another strong crossvein d2, in alignment with d1, is present between CuPaβ and the anal node at the level of the plectrum. The harp is thus located between CuPa, CuPaβ, d2 and PCuA. In observed † Protogryllidae and † Baissogryllidae (see Appendix 3: Figs S5 View FIG ; S 6 View FIG ), we noticed a reduced vein in the harp that could correspond to the CuPb, because of its base connected to that of CuPa. These fossils have two branches of PCu, PCuA and PCuP, with their characteristic strong and curved base ( Fig. 6 View FIG ). While it has been suggested that the † Baissogryllidae and † Protogryllidae may have a file as in modern crickets (i.e., on the highly curved PCu), no stridulatory teeth are visible on photographs or illustrations of fossil specimens that we have studied. Some teeth are however visible on the PCuA in some undescribed baissogryllids from the lower Jurassic of Luxembourg (H. J. and A. N., pers. obs.), supporting this hypothesis, but it cannot be generalized to all baissogryllid and protogryllid fossils. Two anal veins, AA and AP, are located posteriorly. In the † Baissogryllidae that we observed ( Fig. 6C, D, E View FIG ) but not in the † Protogryllidae ( Fig. 6A, B View FIG ), the area between the CuPaα2 and CuPaβ is widened: by its position, this area could correspond to the mirror cell s. str. (mi) of modern crickets, but it is distally opened and not closed by s1 as it is in modern crickets.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.