GRYLLOTALPIDAE, According
|
publication ID |
https://doi.org/ 10.5252/zoosystema2023v45a24 |
|
publication LSID |
lsid:zoobank.org:pub:8D29DD39-7ADC-4B36-8E95-3A3F930301D6 |
|
DOI |
https://doi.org/10.5281/zenodo.10392060 |
|
persistent identifier |
https://treatment.plazi.org/id/FB7687BD-FFD4-C73A-7765-FAD45D9026C5 |
|
treatment provided by |
Plazi |
|
scientific name |
GRYLLOTALPIDAE |
| status |
|
FOREWING VENATION PATTERN IN GRYLLOTALPIDAE View in CoL
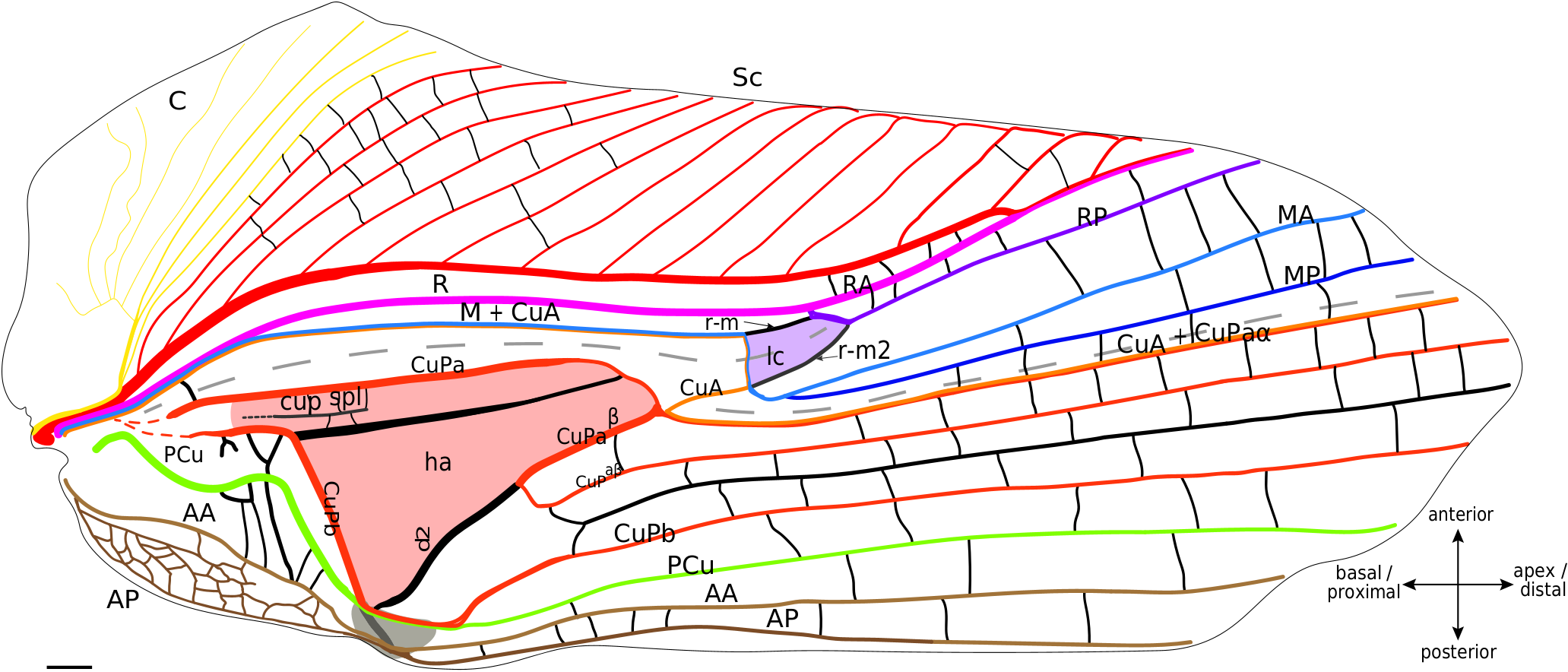
According to our hypothesis, the venation of the lateral field of mole crickets is almost the same as in the crickets s. str. and fossil families. Indeed, even if their bases are very close, we can identify the C, Sc, R and M+CuA ( Fig. 7 View FIG ).
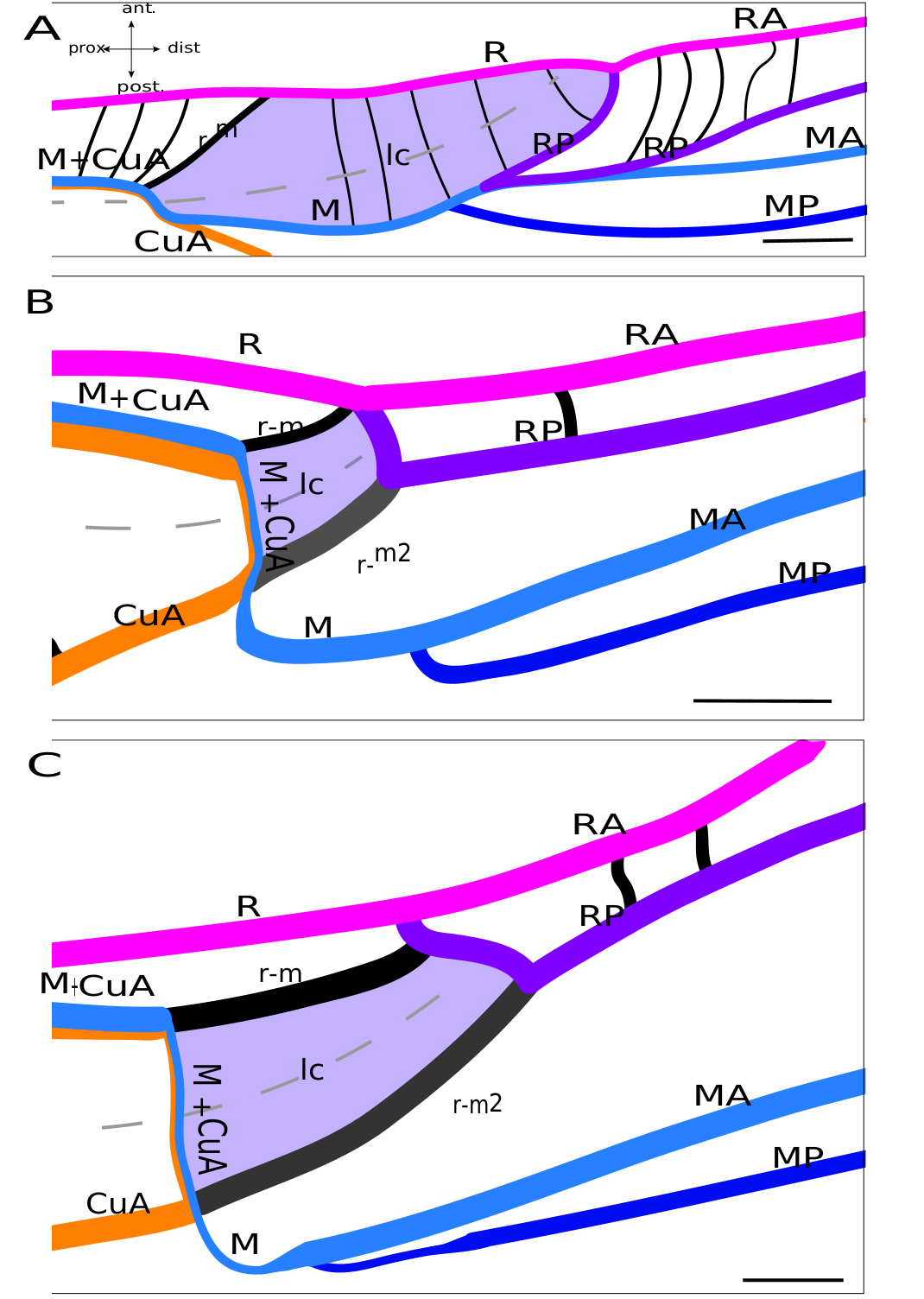
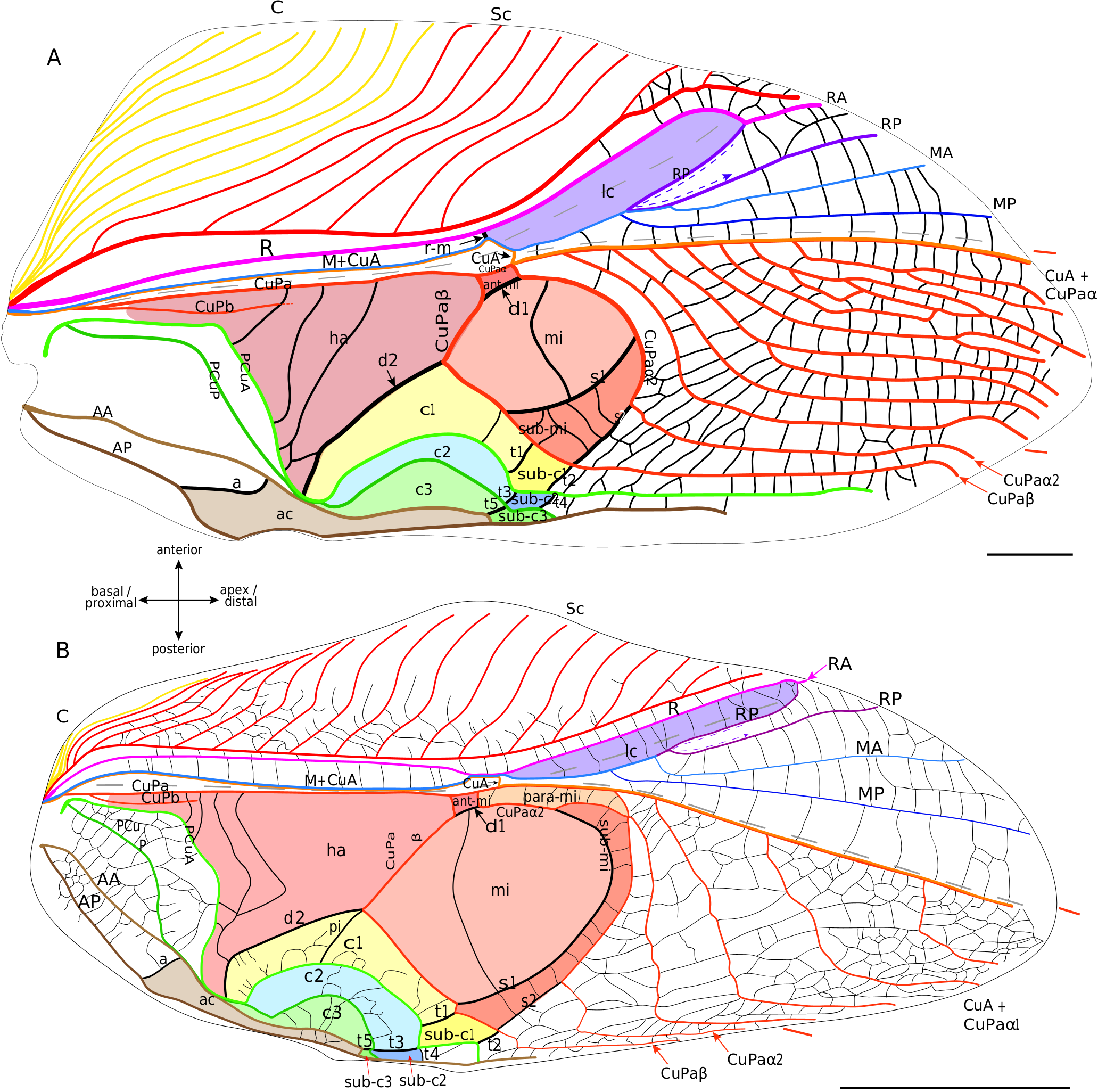
Lateral field. In the proximal half of the wing, Sc, R and M+CuA veins are almost parallel to each other. The C is fused basally with the Sc, and is composed of several slender branches directed anteriorly. Sc has the same branching shape as in Grylloidea , and we also do not distinguish between ScA and ScP in mole crickets. R, posterior to Sc, is strong and convex. M+CuA is the most posterior vein of the lateral field. Four distal branches are usually present in the lateral field and in the so-called ‘flexible zone’ ( Fig. 7 View FIG ): The two most anterior veins are identified as RA and RP, while the other two may be MA and MP. RA and RP are both strong, but the RP appears to be slightly concave compared to the very convex RA. MA is clearly stronger than MP and convex, while MP is concave. Just at the base of the flexible zone, a small cell is visible. According to our hypothesis, this small cell could be homologous to the lanceolate cell of Grylloidea and fossil taxa ( Fig. 8 View FIG ). This interpretation necessitates numerous vein reorganisation (i.e., change of vein polarity and thickness), but it is well-supported by similarities between this cell and the lanceolate cell of fossil crickets (as in † Angarogryllus angaricus , Fig.8A View FIG ), as shown by: the presence of a curved crossvein r-m between R and M; the presence of a fold crossing part of M+CuA after its point of contact with r-m and then crossing this cell; the relative positions of the veins that define the lanceolate cell of crickets; and the relative convexities of the four distal veins of this area (RA/RP and MA/MP). According to this scheme, r-m would have been reinforced in the continuity of the M+CuA in some species as Scapteriscus sp. ( Fig. 8C View FIG ), while the weak transverse vein that borders basally the lanceolate cell would be the ‘real’ M+CuA. But this pattern is not present in all Gryllotalpidae : for example, in Gryllotalpa sp. ( Fig. 8B View FIG ), r-m is slightly oblique (as in some † Protogryllidae ) and weaker than the main veins (except the part of M+CuA that forms the base of the lanceolate cell, which is as weak as in Scapteriscus sp. ). In fact, the polarity and orientation of the veins delimiting this cell are variable according to the species, making the identification of the vein bordering distally the lanceolate cell very complicated: it will be called here ‘r-m2’ because of its relative position ( Fig. 8 View FIG ), but we are aware of three possible interpretations for these veins. A first hypothesis would consider this very strong and convex ‘r-m2’ as another anterior branch of MA, but this would imply a change in convexity distally, when this branch merges with RP (because RP+MA would be concave). Another hypothesis is that ‘r-m2’ is the result of the capture of the base of RP (before its fusion with MA, see in † Angarogryllus angaricus , Fig. 8A View FIG ) by the very distal part of RP (after its separation with MA, Fig. 8A View FIG ), a scheme that could apply to some Grylloidea (like Phyllogryllus sp. , see Fig. 4B View FIG ). Finally, r-m2 could just be a reinforced crossvein between R and M. Clearly, the origin of the ‘r-m2’ vein cannot be determined with certainty according to our observations.
As in crickets, mole crickets have a median fold between M+CuA and CuPa ( Fig. 8 View FIG ). This fold is clearly less marked than in the Grylloidea . We can also notice that two distal folds border the flexible zone: one crossing the lanceolate cell and the other running along CuA+CuPaα.
Dorsal field. From the most anterior to the most posterior, the following veins are present: CuPa, CuPb, a simple PCu, AA and AP ( Fig. 8 View FIG ). CuPa begins with a more or less straight trajectory oriented toward the distal margin; it then curves slightly posteriorly before dividing into an anterior CuPaα and a posterior CuPaβ. CuPaα merges with the CuA at its base. In the observed specimens, CuA+CuPaα is simple. CuPaβ is oblique and bent proximally on its first part, then curves sharply to orient distally and begins a trajectory parallel to the CuA+CuPaα ( Fig. 8 View FIG ). An oblique and strongly reinforced secondary vein d2 is in continuity with the base of CuPaβ and connects it to the anal node ( Fig. 8 View FIG ). CuPb is very blurred at its base, although it originates from the same basivenal bulla as CuPa (see Desutter-Grandcolas et al. 2017). CuPb is strongly curved at right angle and bears teeth on its ventrally side (= stridulatory file). A strong longitudinal vein identified as a secondary vein connects CuPa with CuPb in the harp. On several mole cricket specimens that we observed (as in Scapteriscus sp , Fig. 7 View FIG ; Appendix 3: Fig. S7C View FIG ), we noticed the presence of a short vein that we identify tentatively as a ‘cup spl’ (see Discussion). A large cell is present between d2 and CuPb, posterior to the harp; it is not homologous to the harp, nor to the mirror of the Grylloidea , because it is not delimited by the same veins. We propose to name it the subharp cell. The PCu has a characteristic curved and rounded base. CuPb and PCu merge at the plectrum level, before separating distally to the plectrum and running rather parallel to the longitudinal axis of the wing ( Fig. 8 View FIG ). The AA vein is often well-differentiated from the base, while AP sometimes forms a network of small undifferentiated veins at its base. The anal veins fuse at the level of the plectrum before dividing again distally and running parallel to the longitudinal axis of the wing.
In the distal half of the dorsal field, between the CuA+CuPaα and the posterior margin, vein configuration is highly variable among the species, or even between the two wings of the same species, or the two wings of the same individual (as in Gryllotalpa sp. , Appendix 3: Fig. S7A, B View FIG ). Additional veins, which we consider as intercalary secondary veins, may even be inserted between the main veins ( Fig. 8 View FIG ). However, if vein fusions or branching may vary in this area, all these veins are generally parallel to each other and run parallel to the longitudinal axis of the wing.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |