Grania trichaeta Jamieson, 1977
|
publication ID |
https://doi.org/10.5281/zenodo.189048 |
|
DOI |
https://doi.org/10.5281/zenodo.6217978 |
|
persistent identifier |
https://treatment.plazi.org/id/FB45AF01-C962-876A-80E2-FF54F9BDA75A |
|
treatment provided by |
Plazi |
|
scientific name |
Grania trichaeta Jamieson, 1977 |
| status |
|
Grania trichaeta Jamieson, 1977 View in CoL
( Figs. 8 View FIGURE 8 , 10 View FIGURE 10 E)
Grania macrochaeta trichaeta Jamieson, 1977: 345 View in CoL –347, fig 5, plate 1G. Grania macrochaeta trichaeta View in CoL ; Coates 1984: 46, fig 5A.
New material examined: AMS main coll. W.35554-W.35559, 6 whole-mounted front end specimens, 2 of which are from stn. L16, 2 from L26, and 1 each are from stns. L17 and L22, all voucher specimens for COI barcodes (GenBank accession no’s GQ247640 View Materials , GQ247642 View Materials - GQ247646 View Materials ). SMNH main coll. 105540-105559, 20 whole-mounted specimens from Lizard Island (stns. L1 (9), L16 (11)). SMNH main coll. 105560-105584, 25 whole-mounted specimens from Heron Island (stns. H6 (10), H19 (9), H25 (6)). First author’s collection: 87 specimens from Lizard and Heron Islands (stns. L2 (1), L4 (2), L6 (2), L7 (4), L10 (1), L13 (1), L14 (4), L15 (3), L17 (1), L22 (4), L23 (1), L26 (2), L31 (7), L32 (1), H2 (1), H5 (1), H7 (4), H9 (1), H10 (7), H11 (2), H12 (1), H13 (2), H14 (1), H15 (1), H16 (1), H17 (1), H18 (4), H20 (1), H21 (9), H22 (1), H23 (2), H24 (13)).
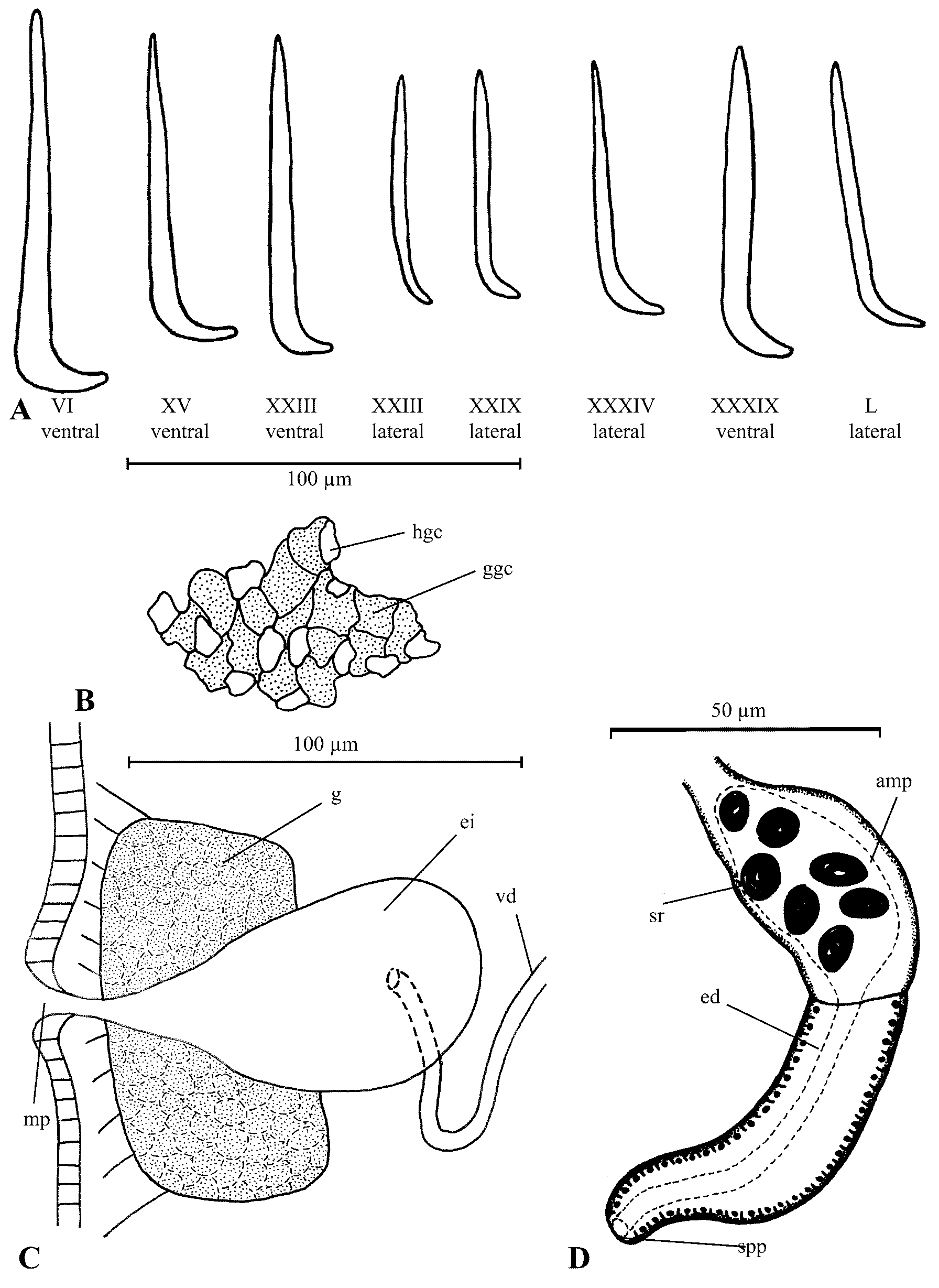
Description of new material: Living specimens white. Body 7.5–13.3 mm long, 0.14–0.24 mm wide at III, 0.14–0.25 mm at clitellum (n=30). Segment number 43–56 (n=30). Prostomium rounded, 75–115 µm wide, 55–80 µm long (n=30); prostomial epidermis 18–26 µm thick dorsally, 18–25 µm anteriorly, 9–17 µm ventrally (n=30). Peristomium 110–185 µm wide at 1/2 (n=30). Ventral chaetae commencing in V; lateral chaetae commencing in XX–XXIII. Chaetae larger anteriorly; 75–95 µm long in pre-clitellar segments, 60–75 µm long near posterior body end; chaetal shaft cylindrical proximally, tapering distally; entally bending into a slender foot, 12–20 µm long (n=30), with low instep, curved sole and no heel ( Figs. 8 View FIGURE 8 A, 10E); chaetal index=4.17, n=30, sd=0.538. Epidermal gland cells inconspicuous. Clitellum 13–18 µm thick, extending from anterior of XII to mid XIII, with an irregular pattern of granular gland cells interspersed with hyaline cells at a frequency of about 4:1 ( Fig. 8 View FIGURE 8 B), except near male pores where hyaline cells are absent, and midventrally, where no gland cells are present. Copulatory gland not observed in XIV. Spermathecal pores lateral, located right behind 4/5. Male pores located ventrolaterally in mid XII.
Brain posteriorly indented. Head organ absent. Pharyngeal glands in IV–VI, not united dorsally; dorsal lobes present in IV–VI, ventral lobes present in IV (1 pair), V (2 pairs) and VI (2 pairs); not connected dorsally. First pair of nephridia at 7/8. Dorsal blood vessel commencing in XVIII–XXI. Chloragogen cells small (5–7 µm tall). Coelomocytes oval, granular, present mostly posterior to clitellum, 11µm across at the widest point. Sperm sac extending posteriorly from clitellum as far back as XVII. Sperm funnels of uniform width, 60–65 µm wide, twice to three times as long as wide. Heads of spermatozoa 14–17 µm long. Vasa deferentia unmodified, loosely coiled in XII–XIII, 5 µm wide, internally ciliated. Penial apparatuses ( Fig. 8 View FIGURE 8 C) with uniform oval glandular structures, 75–100 µm long, 40–50 µm wide, next to 70–120 µm deep epidermal invaginations from male pores; vasa deferentia opening into invaginations; stylets absent (penial bulb type 3). Egg sac reaching as far back as XXI. Spermathecae ( Fig. 8 View FIGURE 8 D) attached to oesophagus near 5/6; ampullae pearshaped, 30–40 µm in diameter, ectal duct of nearly uniform width, slightly attenuated at both ends, 50–60 µm long and 15–20 µm wide; 6–8 sperm rings per spermatheca; no glands at spermathecal pores.
Remarks: Jamieson (1977) originally described this species as a subspecies of G. macrochaeta ( Pierantoni, 1901) , but as mentioned above (see Introduction), we argue that separate species status is warranted here. In his description, Jamieson placed much focus on the distribution of the ‘dorsal’ (= lateral) chaetae, which allegedly occurs as only one per segment in XXI–XXX or so, then occur at both positions (i.e. right and left) from XXX to XL, only to revert back to being only 1 or even 0 per segment in the posteriormost segments. In all specimens studied here, however, the lateral chaetae commence in XX–XXIII at both positions and are regularly distributed, except in a few places where the worm might have been damaged. The lack of a chaeta at one position or another is rather common in both live and mounted Grania specimens, due to damage or random variation. This may have caused the pattern observed by Jamieson.
More dependable species characteristics than the occasional absence of lateral chaetae would be the shape of the spermathecae, chaetae and penial bulbs. The material examined here conforms completely to that described by Jamieson with regards to the spermathecal structure. The chaetae were originally described as occurring ventrally in V and all segments posterior to V in most cases (VI in some), and from XXI–XXIX (most often XXI) laterally. The material studied here also conforms to this description. The chaetal structure is also similar in general shape, although the drawings made by Jamieson indicate thicker chaetal shaft than found in this study. The structure of the penial apparatuses was not described by Jamieson, but later by Coates (1984); in all specimens studied here, the bulbs are highly similar to that described by Coates. Furthermore, there does not seem to be any significant differences between specimens from the two sites of Lizard and Heron Island, neither in body dimensions, chaetal distribution, penial apparatus nor spermathecal shape, an indication that these two populations are, or recently were, connected. No molecular data is currently available from the population at Heron Island, however.
Morphologically, G. trichaeta is similar to G. hongkongensis Erséus, 1990 in chaetal distribution and shape, as well as spermatecal shape. The penial bulb of G. hongkongensis , however, was described as a “ type 1” sensu Coates, 1984, which is characterized by vasa deferentia opening into very small epidermal sacs inside the male pores ( Erséus, 1990). Nevertheless, there is an invagination present in G. hongkongensis , albeit small. As described by Coates (1984), penial bulbs type 1 and 3 only differ in the size of the epidermal invaginations (in type 3 bulbs, the invaginations form distinct lateral sacs), a character which could easily change from one state to another over evolutionary time.
A species recently described from New Caledonia, G. f u s t a t a De Wit & Erséus, 2007 is also morphologically similar to both G. trichaeta and G. hongkongensis , which suggests a close relationship. Grania fustata possesses penial apparatuses similar to those of G. trichaeta , with large epidermal invaginations, and chaetae similar to those of G. trichaeta , although larger. The spermathecae of G. f u s t a t a differ from those of G. trichaeta , however, in having bipartite ectal ducts, with the outermost part bulbous in shape. Furthermore, G. f u s t a t a has the clitellar gland cells arranged in regular rows, as opposed to those of G. trichaeta , which are irregularly distributed.
Distribution and habitat: Lizard Island (new record) and Heron Island, Great Barrier Reef, subtidal (to 7 m), heterogeneous sand. Found at all sampling dates and in great numbers in the study area.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Grania trichaeta Jamieson, 1977
| Wit, Pierre De, Rota, Emilia & Erséus, Christer 2009 |
Grania macrochaeta trichaeta
| Coates 1984: 46 |
| Jamieson 1977: 345 |