Adeonella calveti Canu and Bassler, 1930
|
publication ID |
https://doi.org/10.1080/00222931003760061 |
|
persistent identifier |
https://treatment.plazi.org/id/FB2D9A7D-A449-EE2D-FE5B-FE3878DFAC84 |
|
treatment provided by |
Felipe |
|
scientific name |
Adeonella calveti Canu and Bassler, 1930 |
| status |
|
Adeonella calveti Canu and Bassler, 1930 View in CoL
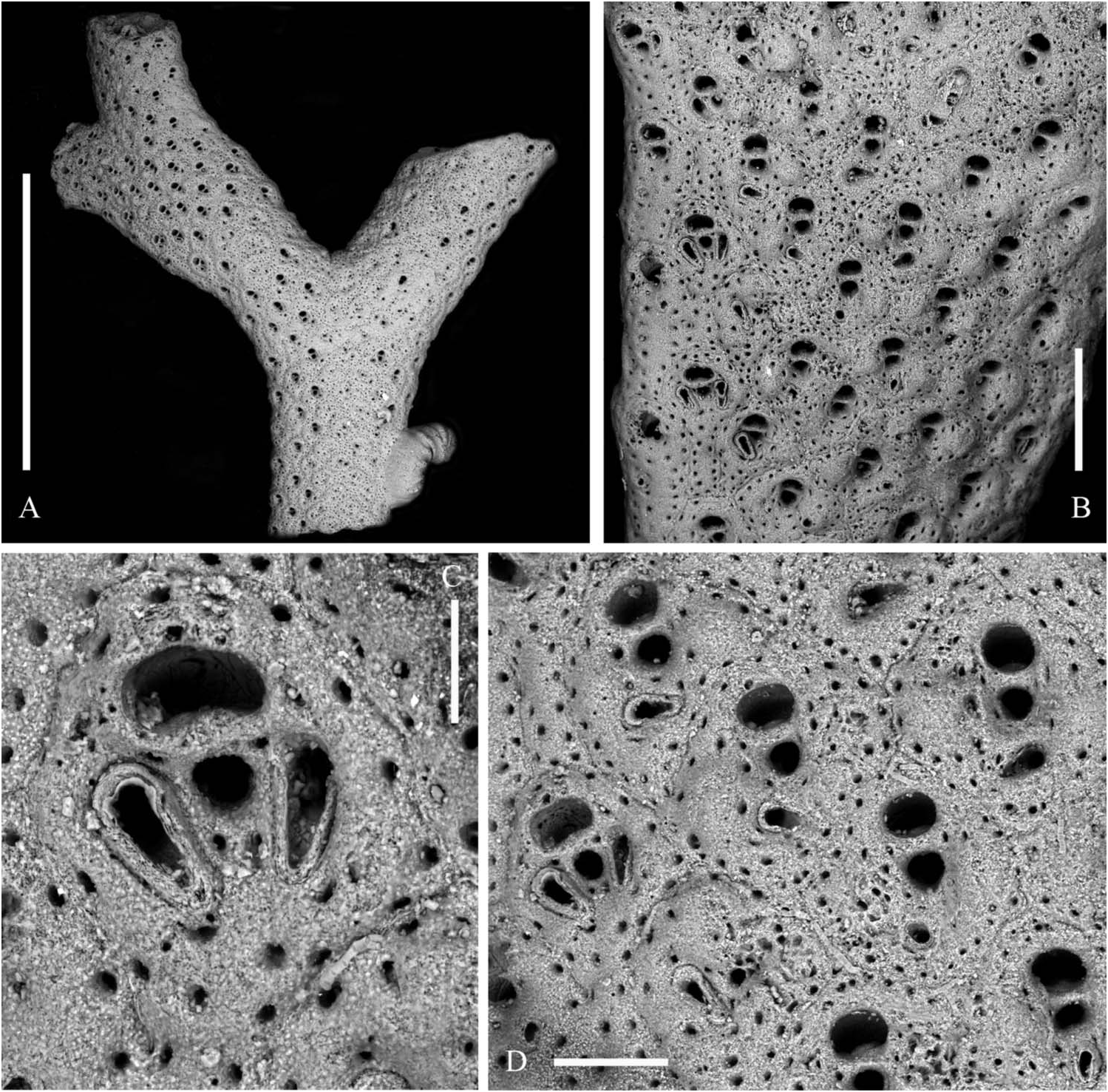
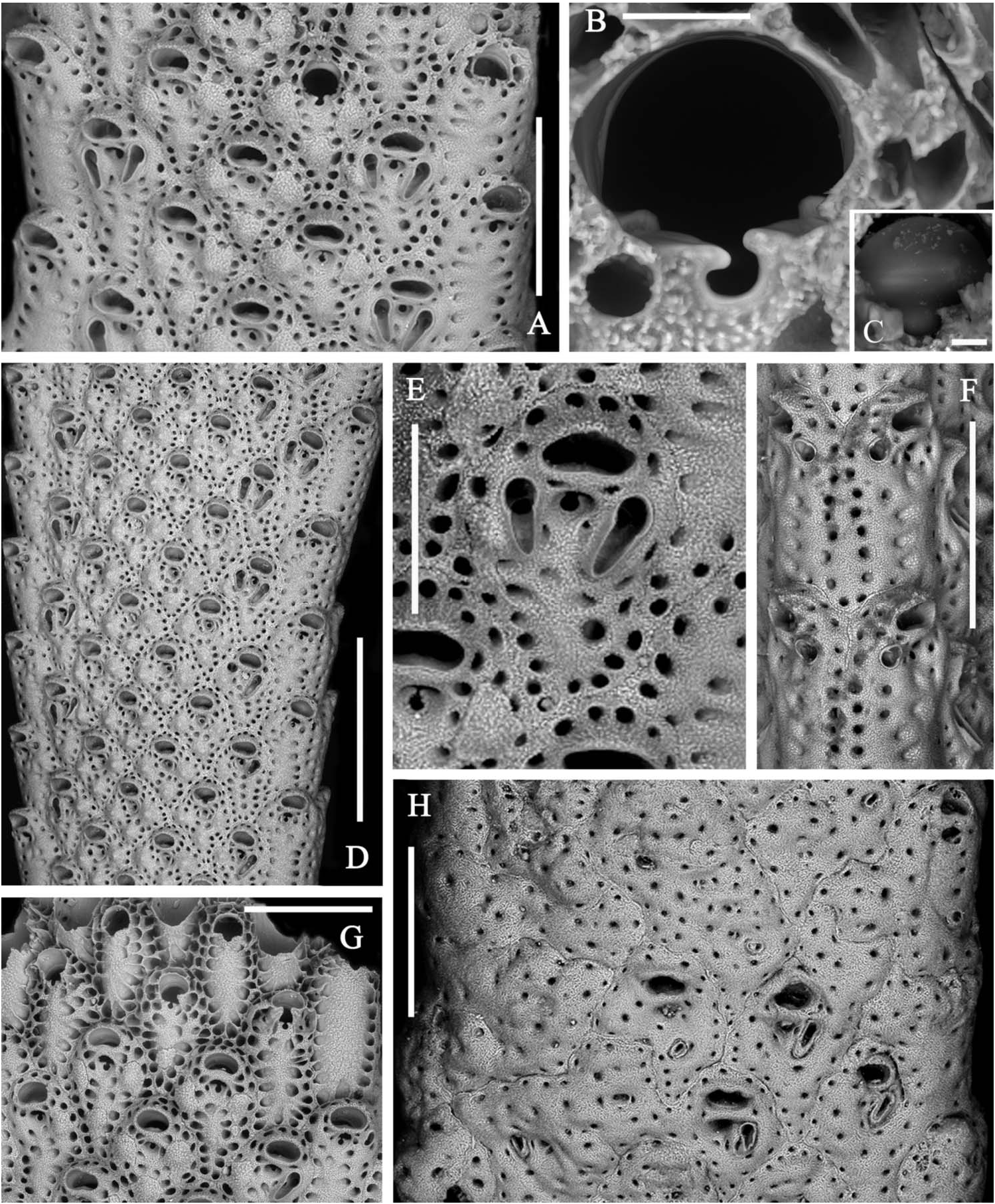
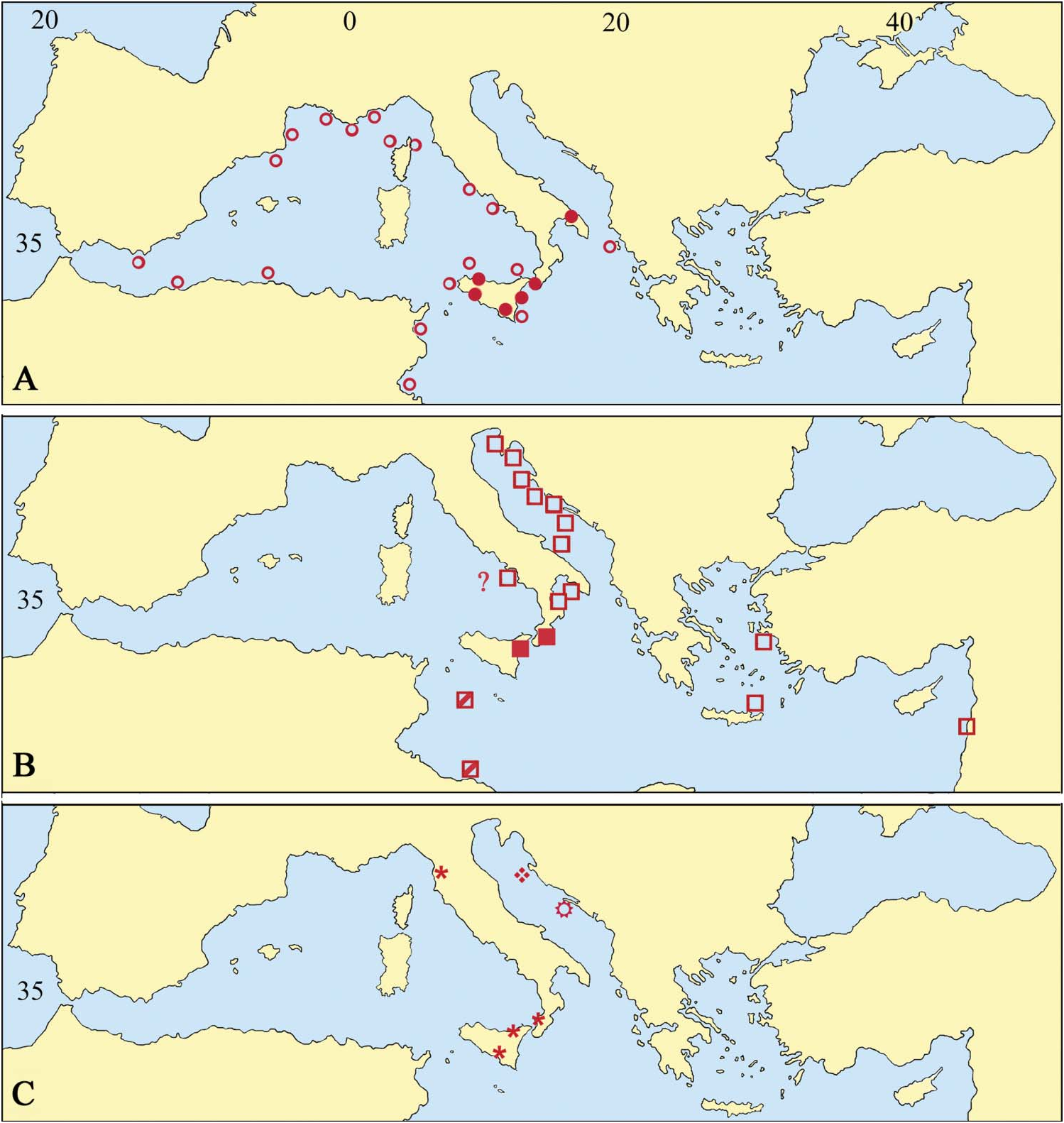
( Figures 1A–D View Figure 1 , 2 View Figure 2 , 3 View Figure 3 , 4A View Figure 4 )
Adeonella calveti Canu and Bassler, 1930: 68 View in CoL , pl. 10, figs. 1–4.
Adeonella pectinata f. africana Calvet, 1903: 33, pl. 2, fig. 4.
Adeonella calveti: Gautier, 1962 View in CoL , 220; Zabala, 1986: 389, fig. 125; Hayward, 1988: 173, fig. 23 A,B; Zabala and Maluquer, 1988: 144, fig. 359; Reguant and Maluquer, 1992: 150, pl. 2, fig. 6; Rosso, 1996: 211, fig. 5d.
Adeonella polystomella ( Reuss, 1848) View in CoL : Poluzzi and Rosso, 1988: 91, pl. 3, fig. 2; Pouyet and Moissette, 1992: 73, pl. 11, fig. 6.
Examined material
Holocene. Lectotype: MNHN: BP-A73_01. Ionian Sea , Eastern Sicily : Gulf of Noto ( PS/81 cruise: isolated to hundreds of dead fragments from several stations: 31–83 m, DC and 84–128 m, DL); Gulf of Catania ( LCT/80 , sample 69: numerous dead fragments: 90 m, DL); Ciclopi Islands (Ciclopi 2000 cruise: isolated to few hundreds of dead fragments from several stations: 43–62 m, DC, and 83–95 m, DL); off Taormina: two large living colonies from about 40 m; off Cape S. Alessio (Urania 1993 cruise: a few dead fragments: 208 m). Sicily Straits: Egadi Islands (a few living specimens from off Marettimo, 19 m, C); Graham Bank (CR/90 cruise: several dead fragments in subsurface sediments from some stations, 97–210 m, DC and DL mixed with RL and VP). Messina Straits (Urania 1997 cruise: a very few worn fragments from several stations: 90–341 m, displaced in deep-water dune fields; MERC cruise 2006: few dead, seemingly displaced, fragments from some stations: 136–451 m). Tyhrrenian Sea: Ustica (Banco Apollo: some living and dead fragments, 60 m, C and SGCF; Secchitella: a few living and dead fragments: 80 m, DC; Faro I: a few dead fragments: 98 m, DL); Ponza Island (Min ‘89/207: a few dead fragments: 84 m, DC); Corsica (off Calvì: BRACORS 1983 cruise: some dead fragments from a few stations: 110–150 m, DL); north-west Sardinia ( Galatea cave : four living colonies: 7 m, GSO). All these specimens are deposited as PMC. R.I.H. B5a. Pleistocene: Würmian: off Acitrezza, Ciclopi Islands : a few fragments. Tyhrrenian: few specimens from Madonna di Adonai , south-east Sicily. Early Pleistocene: a single specimen from the “Case Catarinicchia” section, west Sicily, uncertainly referred to this species and seemingly displaced; Furnari (Messina, north-east Sicily): a single specimen from a deep-water rubble-pocket along a fault scarp. All these specimens are deposited as PMC. R. I.Ps. B5b. Middle-to-Late Pliocene: several specimens from inferred circalittoral environments in sandy layers cropping out near Altavilla (Palermo, west Sicily). PMC. R. I.Pl. B5c.
Description
Colony ( Figure 1A–D View Figure 1 ) orange to salmon in colour, erect, rigid forming delicate, roughly hemispherical pillows up to 12 cm high and 15 cm in diameter in the available material. Colonies seem to be formed by a series of irregular fans consisting of straight to gently curving ribbon-like, branches usually bifurcating in the same plane but repeatedly twisting at about 90° to give rise to further fans. Anastomoses nearly absent, branches usually frontally curving, tilting and twisting to avoid contacts with their neighbours. Branches with regularly and slightly serrated outlines, usually 0.5–0.8 mm thick and 2–3 mm wide, but reaching 4 mm immediately before bifurcations and in the very basal portions, sometimes becoming nearly cylindrical owing to secondary calcification.
Zooids arranged in two back-to-back layers and in four to nine alternating longitudinal rows on each side, the marginal rows distinctive ( Figure 3A,D View Figure 3 ). Autozooids separated by grooves and a thin raised sutura. Autozooids dimorphic because of their size and shape and the presence and morphology of peristomial and frontal avicularia. Zooids ovoid to markedly rhomboid in early ontogeny. Marginal zooids slightly longer and larger than median zooids but subrectangular in shape and, consequently, with a markedly larger surface, forming obvious lateral rows. Frontal wall finely granular with a peripheral row of relatively large, densely spaced pores. A second inner row is present, obvious on the marginal zooids but becoming barely distinguishable on the median zooids. Two latero-oral and one proximal frontal knob typically develop on the median zooids, progressively swelling with ontogeny and becoming encircled by pores ( Figure 2A,G View Figure 2 ). Primary orifice nearly as long as wide, with a semicircular distal rim and a straight proximal lip; sinus U-shaped, occupying nearly one-third of the entire width, suddenly reduced to a nearly dropshaped notch by the convergent growth of two lateral wings of frontal calcification ( Figure 3B,C,G View Figure 3 ); condyles deeply inclined laterally, robust and round edged. Secondary orifice formed suddenly through the development of a slender bridge of calcification, distally raised and sometimes tubular, semicircular to transversely elliptical, the proximal border sometimes slightly convex. Spiramen subcircular and small, located just proximal to the peristome leaving visible the orificial dropshaped sinus in all but latest ontogenetic stages. Peristomial avicularium lateral to the spiramen, single or paired, often lacking on several zooids; typically with a subtriangular rostrum and without cross bar, small-sized and disto-medially directed on marginal zooids ( Figure 3F View Figure 3 ); straight to slightly curved, elongated triangular and without crossbar, decidedly larger, proximally or proximo-medially directed on median zooids ( Figure 3A,D View Figure 3 ). Additional small subtriangular, randomly oriented avicularia can be present on the frontal surfaces of marginal zooids and rarely on the median zooids, in late ontogenetic stages and mostly after orifices, spiramina and peristomial avicularia had became occluded and completely immersed in secondary calcification ( Figure 3H View Figure 3 ). Vicarious avicularia and gonozooids absent. Adhesion to the substratum through an encrusting base formed by polygonal kenozooids marked by a row of peripheral pores and sporadically exhibiting small immersed subtriangular avicularia.
Measurements
ZL: 545 ± 43.84, 438–621 (3, 20); ZW: 375 ± 37.70, 309–445 (3, 20); mZL: 634 ± 67.20, 539–693 (2, 4); mZW: 325 ± 39.55, 276–366 (2, 4); sOL: 88 ± 8.29, 75–105 (2, 20); sOW: 133 ± 9.39, 115–152 (2, 20); czAL: 166 ± 16.92, 132–206 (2, 20); czAW: 64 ± 5.60, 55–80 (2, 20).
Remarks
Specimen illustrated here as lectotype ( Figure 2A–D View Figure 2 ) is the largest, twice branching, fragment with adult zooids, selected from the four specimens in the collection of Canu and Bassler indicated as the type series and housed at the Natural History Museum in Paris .
Although having some similarities with other Adeonella species , A. calveti is easily distinguishable by the usually paired long triangular, proximally directed avicularia which are characteristically located on median but usually not very central zooids. The species has been sometimes synonymized with, or considered as the living counterpart of (see Gautier 1962), or simply reported as Adeonella polystomella (e.g. Pouyet and Moissette, 1992). With this species A. calveti shares the presence of frontal avicularia in some zooids and the morphology of the primary orifice in some populations from West Africa (see Hayward 1988, fig. 22C). Nevertheless, A. calveti differs from A. polystomella in several features, among which the number, morphology, size and distribution of both peristomial and frontal avicularia (see Gautier 1962: 220) and the secondary orifice, which is more prominent and less compressed. Furthermore, A. calveti has also been considered as a synonym of A. pallasii by Buge and Debourle (1977), but this latter species does not possess elongated longitudinally placed avicularia and has slightly smaller zooids with small-sized paired peristomial avicularia and a different primary orifice (see below). Furthermore, the Z-SI index ( Table 1) is markedly higher for A. calveti , pointing to more elongated zooids whereas the O-SI index is lower, indicating proportionally wider but shorter secondary orifices.
Variability and ecology
As already remarked by Gautier (1962), A. calveti shows wide variability during ontogeny mostly as the result of frontal secondary calcification leading to the complete dipping and obliteration of secondary orifices, ascopores and even the peristomial avicularia. Zooids near the base appear as sieve-like polygonal surfaces, sometimes exhibiting one or a few variously oriented, often small-sized and raised avicularia. In contrast, primary orifices are visible only in zooids of the distalmost rows at growing tips ( Figure 3G View Figure 3 ).
Zooidal dimensions appear slightly greater than those reported by Hayward (1983) for populations from the Gulf of Naples. In particular, a certain difference exists between the Banco Apollo ( southern Tyrrhenian Sea) specimens, whose measurements are similar to those reported by Hayward (1983), and the eastern Sicily shelf ( western Ionian Sea ) specimens, which have the largest zooids, secondary orifices and avicularia .
Also colony morphology is highly variable. Populations from GSO environments form smaller colonies (up to 7 cm high and 4 cm wide in the present material), which are arborescent but more sparsely branched ( Figure 1D View Figure 1 ). In contrast, colonies encrusting tubular substrata, such as tubes of the polychaete worm Sabella , tend to form densely branched subspherical colonies.
This species has been typically recorded from coralligenous bottoms and from semi-dark cave walls (GSO biocoenosis) by Harmelin (1976) and was consequently included within his coralligenous stock. Furthermore, A. calveti is one of the few erect species able to colonize coarse soft bottoms because it has been subordinately found also within shallow (less than 100 m deep) detritic bottoms where DC ( Harmelin 1976) or SGCF ( Di Geronimo et al. 1990) biocoenoses develop. This two-fold ecological distribution, first remarked on by Harmelin (1976), is supported by the present analysis. From the above reported data and observations by Gautier (1962), the depth distribution of A. calveti ranges from a few metres in caves and overhangs, down to 100 m, with an optimum in the shallow circalittoral (midshelf) at about 30– 60 m.
Colonies collected from eastern Sicily shelf bottoms reach large sizes and develop lace-like hemispherical colonies, which form (mostly in their basal parts) the substratum for several taxa among which hydrozoans, serpulids, spirorbids, sponges, foraminiferans and other bryozoans are common. It is noteworthy that spirorbids are almost invariably located on the frontal surface of lateral zooids along the narrow sides of the ribbon-like branches, as also happens on the holotype ( Figure 2A View Figure 2 ). Among bryozoans erect flexible species, mostly represented by crisiids such as Filicrisia geniculata (Milne-Edwards, 1838) , Crisia fistulosa Heller, 1867 , Crisia pyrula Harmelin, 1990 and some Scrupocellaria species , adhering only through rhizooids, are present, together with erect rigid species developing small encrusting basal expansions, such as Reteporella grimaldi (Jullien, 1903) and Idmidronea triforis ( Heller, 1867) . Encrusters are subordinate, mostly represented by the stolonate uniserial runner Aetea truncata (Landsborough, 1852) , and small domiform colonies of Celleporina mangnevillana (Lamouroux, 1816) . In contrast, among the rare multiserial encrusters, Escharella variolosa (Johnston, 1838) is very common, often forming large colonies completely enveloping some branches.
Distribution
First described from the south-western Mediterranean (coasts of Tunisia: Canu and Bassler, 1930), A. calveti has been recorded mostly in the western Mediterranean ( Figure 4A View Figure 4 ), including both the northern and the southern sides ( Gautier 1962 and references therein), Catalonia ( Zabala 1986) and the Alboran sea (Cape of Gata, 50–60 m, rocks with coralligenous and detritic sands: Harmelin et al. 1989), and also the Provencal basin ( Harmelin 1976) and the Tyrrhenian Sea ( Calvet 1903; Hayward 1983, 1988; Di Geronimo et al. 1990). The easternmost record is from Corfù island in the north-east Ionian Sea ( Gautier 1962). Similarly, dead specimens have been invariably recorded from shelf bottoms of southern Spain, in the Alboran Sea (Reguant and Maluquer 1992), and Sicily (Poluzzi and Rosso 1988; Rosso 1989 a, 1996 and unpublished data; Di Geronimo et al. 1990, 1993). Consequently, A. calveti can be considered as a western Mediterranean endemic species, as suggested by Hayward (1983), although extending to the central part of the basin.
Adeonella calveti View in CoL was virtually unknown as a fossil (see Gautier 1962; Hayward 1988) before some recent records ( Di Geronimo et al. 1994, 1997; Coppa et al. 2001) from Pleistocene sediments of western Sicily, Calabria and Apulia, respectively. Nevertheless, the species actually has a wider stratigraphical distribution because it is present in the Middle–Late Pliocene sediments cropping out at Altavilla, near Palermo in western Sicily (Rosso, personal data) from where it was recorded as A. polystomella View in CoL by Pouyet and Moissette (1992: 73; pl. 11, fig. 6). Adeonella calveti View in CoL was presumably widespread and relatively common also in the Pleistocene in the same area because it has been found in other localities of southern Calabria and some
formation of the orificial bridge concealing the primary orifice. Scale bar: 500 µm. (H) A near-base branch portion. Note the increase of frontal calcification progressively occludes orifices, ascopores and avicularia, and zooids become indistinct and often coalesce to form wide, irregularly-shaped plaques, often equipped with small irregularly located triangular avicularia. Scale bar: 500 µm.
Notes: Acronyms as listed in Materials and methods. areas in western and eastern Sicily (Rosso, personal data). Further specimens come from the Tyrrhenian sediments cropping out near Syracuse, the submerged Würmian deposits off Acitrezza in the Gulf of Catania (Rosso, personal data) and the post-Würmian submerged sediments off Sciacca ( Sicily Straits: Di Geronimo et al. 1993). Of note is that the fossil and present-day geographical distributions of A. calveti largely overlap, pointing to a presumed persistence of an endemic distribution through time. Nevertheless, interpretation is complicated by the confusion of A. calveti with both A. polystomella and A. pallasii (see below).
| MNHN |
Museum National d'Histoire Naturelle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Adeonella calveti Canu and Bassler, 1930
| Rosso, Antonietta & Novosel, Maja 2010 |
Adeonella calveti : Gautier, 1962
| Rosso A 1996: 211 |
| Hayward PJ 1988: 173 |
| Zabala M 1986: 389 |
Adeonella pectinata
| Calvet L 1903: 33 |