Balcha Walker
|
publication ID |
https://doi.org/ 10.11646/zootaxa.1033.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:E1D64688-2A79-49B9-B71E-B47CFD9D2DA5 |
|
persistent identifier |
https://treatment.plazi.org/id/FA057931-513D-FFC6-FE99-FD9079DE74B5 |
|
treatment provided by |
Felipe |
|
scientific name |
Balcha Walker |
| status |
|
Balcha Walker View in CoL View at ENA
Balcha Walker, 1862: 394 View in CoL . Type species: Balcha cylindrica Walker View in CoL , by monotypy.
Elemba Cameron, 1908: 151 . Type species: Elemba levicollis Cameron , by monotypy. Synonymy by Hedqvist, 1961: 109.
Sauteria Masi, 1927: 333–334 . Type species: Sauteria eximia Masi , by original designation. Synonymy by Bouček, 1988: 544.
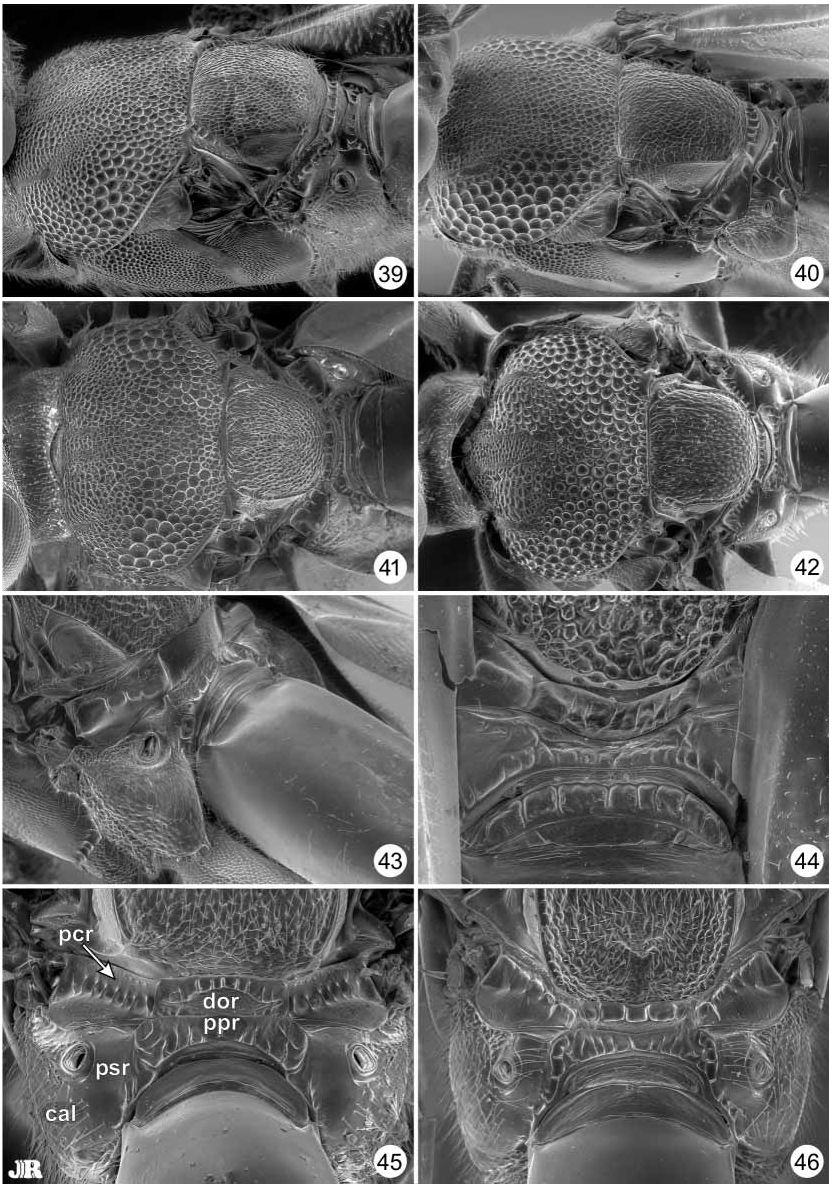
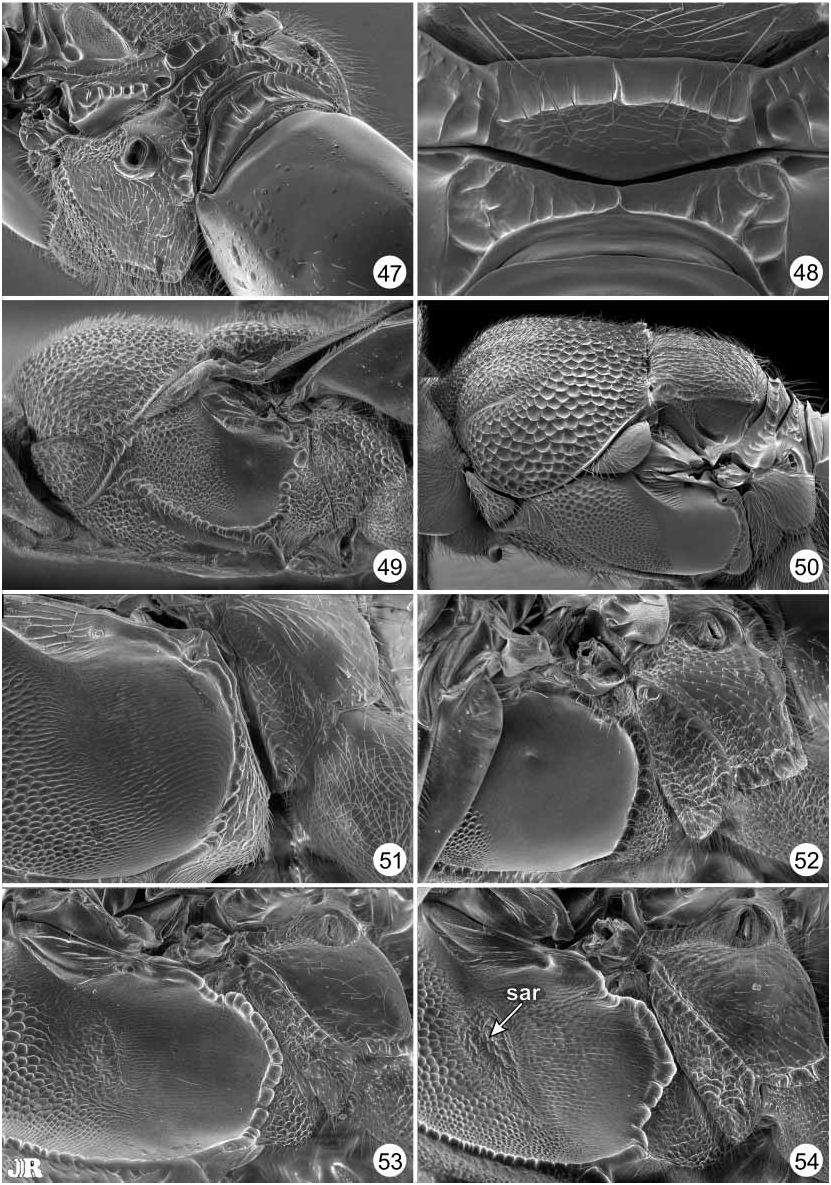
Diagnosis. Antenna 13segmented; flagellum with Fl 2 distinctly longer than pedicel and often distinctly longer than clava; clava 3segmented. Mesoscutum in anterior half with submedian, linear, parallel notauli and similar parapsidal lines paralaterally ( Figs. 31–42 View FIGURES 31–38 View FIGURES 39–46. 39–42 ); usually patterned by comparatively finely sculptured and darker colored regions relative to more grossly sculptured and brilliantly metallic regions (cf. Figs. 9–18 View FIGURES 9–18 and 31–42 View FIGURES 31–38 View FIGURES 39–46. 39–42 ). Scutellum quadrate, with lateral margin carinate for entire length; axilla elongatetriangular to almost linear along extreme anterolateral corner of scutellum ( Figs. 31–38 View FIGURES 31–38 ). Prepectus small, the apex distinctly separated from base of tegula even when mesonotum not highly arched and prepectus extended horizontally (Figs. 7, 8, 49, 50). Acropleuron and metapleuron separated by only very slender region dorsally but by triangular, setose, lower mesopleuron ventrally ( Figs. 49–54 View FIGURES 47–54. 47 ); acropleuron conspicuously punctatealveolate anteriorly and more finely though variably sculptured posteriorly (Figs. 7, 8, 49–54). Protibia without dorsal spicules. Mesotarsus with single row of pegs along both ventral edges. Gaster with Gt 7 and Gt 8 fused into syntergum.
Distribution. Balcha occurs naturally in the Afrotropical, Australasian, Oriental and Palearctic regions. Although B. indica (Mani & Kaul) is present in the Nearctic region, this is presumed to have resulted from accidental introduction (see species discussion). Species are not known from Australia, but species diversity is greatest in the Australasian and Oriental regions, particularly southeast Asia to Papua New Guinea. The most northern record is for B. reticulata (Nikol’skaya) from the Russian Far East, near Vladivostok.
Remarks. Balcha can be distinguished from other genera of Calosotinae using the key to world genera and generic description given in Gibson (1989), and from other eupelmid genera in North America using the key of Gibson (1997). The genus has been treated as a synonym of Eusandalum Ratzeburg ( Ashmead 1904; Risbec 1952; Hedqvist 1961), Polymoria Förster ( Hedqvist 1970) and Calosota ( Bouček 1988) , but was reestablished as a valid genus by Gibson (1989). Gibson (1989) supported monophyly of the genus by a single putative autapomorphy, the unique mesoscutal color and sculpture pattern of individuals. All species then known shared a mesoscutum that was conspicuously patterned by grossly umbilicate and brilliantly colored regions contrasting with more reticulatepunctate, darkly colored regions. However, the newly described species B. punctiscutum has the mesoscutum entirely punctate ( Figs. 32 View FIGURES 31–38 , 49 View FIGURES 47–54. 47 ) even though it has a characteristic bicolored color pattern (Fig. 5). Gibson (1989) hypothesized that Balcha is most closely related to Calosota Curtis and Tanythorax Gibson based on shared presence of parallel notauli [ Gibson (1989), state 7(4)], an entirely carinate lateral margin of the scutellum [state 21(2)], a mesopleuron composed of an almost completely enlarged acropleuron and a convex mesepimeron on the same plane as the acropleuron [state 3(3b)], and possibly by presence of a 3segmented clava [state 2(3)], though polarity of the latter character is ambiguous. Balcha and Tanythorax differ from Calosota by having a small prepectus that does not extend to the tegula (Figs. 7, 8, 49, 50), which could support a sistergroup relationship between the two taxa ( Gibson 1989, fig. 1a). However, a small prepectus is possessed also in Calosotinae by Chirolophus Haliday , Licrooides Gibson and some Eusandalum ( Gibson 1989) . Members of these last three genera have V shaped notauli ( Gibson, 1989, figs. 67, 68; tables 1, 2) and a reduced prepectus likely was derived at least twice in Calosotinae based on postulated relationships among the genera ( Gibson 1989, fig. 1). Although Gibson (1989) suggested that both Balcha and Tanythorax likely are monophyletic, he stated that Calosota could be paraphyletic relative to one or both taxa. If anything, results of this study further suggest the possible paraphyly of Calosota relative to Balcha (see below). However, Balcha comprises a relatively speciose group of at least 16 species that can be differentiated from Calosota based on a small prepectus and from Tanythorax based on coarse mesosomal sculpture. Until exact relationships among the species of the taxa can be demonstrated I prefer to treat the assemblages as separate genera.
Species groups. The 16 recognized world species of Balcha are subdivided into four species groups based on structural and setal features to facilitate species comparisons. The cylindrica group, composed of B. cylindrica , B. indica and B. reburra n. sp., may comprise a monophyletic assemblage based on shared presence of setae on the posterior, coriaceous surface of the dorsellum ( Fig. 48 View FIGURES 47–54. 47 ), though this group likely renders the laciniosa group paraphyletic (see below). The anemeta group consists of B. anemeta (Walker) , B. levicollis , B. punctiscutum n. sp. and B. reticulifrons n. sp., and is also distinguished by a unique feature within the genus, the presence of a thin dorsellum ( Figs. 43, 44 View FIGURES 39–46. 39–42 ). Other species in the genus have a thick dorsellum because dorsally there is a short, horizontal, crenulate surface ( Figs. 45–48 View FIGURES 39–46. 39–42 View FIGURES 47–54. 47 ). However, the thin dorsellar structure that characterizes the anemeta group is almost certainly symplesiomorphic rather than synapomorphic because this structure is shared also with species of Calosota and Tanythorax ( Gibson 1989, figs. 53, 57). Females of B. punctiscutum have a uniformly punctate mesoscutum ( Fig. 32 View FIGURES 31–38 ) rather than the partly alveolate or punctatealveolate mesoscutum that characterizes other members of Balcha ( Figs. 31, 33–42 View FIGURES 31–38 View FIGURES 39–46. 39–42 ), as well as having a comparatively less enlarged acropleuron relative to other Balcha species (cf. Figs. 49, 50–54 View FIGURES 47–54. 47 ). Furthermore, B. reticulifrons has the face reticulate (Fig. 4) rather than punctate to punctatealveolate as for other members of the genus (Figs. 1–3, 19–30). A less enlarged acropleuron is hypothesized as plesiomorphic within Eupelmidae and Calosotinae ( Gibson 1989) , but a uniformly punctate mesoscutum or a reticulate face could either represent autapomorphies within Balcha or uniquely retained symplesiomorphies inherited from some Calosota like ancestor. The remaining species of the genus are divided into two species groups based on setal patterns of the propodeal paraspiracular region. The elegans group is composed of B. dictyota n. sp., B. elegans (Masi) , B. eximia and B. eximiassita n. sp., and is distinguished by a setose paraspiracular region ( Figs. 46 View FIGURES 39–46. 39–42 , 47 View FIGURES 47–54. 47 ), whereas the laciniosa group, composed of B. camptogastra n. sp., B. enoptra n. sp., B. laciniosa n. sp., B. reticulata and B. splendida (Girault) , is distinguished by a bare paraspiracular region excluding any setae along its anterior margin ( Fig. 45 View FIGURES 39–46. 39–42 ). Within the anemeta group, females of B. anemeta and B. levicollis share a setose paraspiracular region with elegans group species, whereas B. punctiscutum and B. reticulifrons share a bare paraspiracular region with cylindrica and laciniosa group species. Known Tanythorax have a bare paraspiracular region, but the groundplan state for Balcha is uncertain because both states occur in Calosota . The presence of both propodeal setal states in the anemeta group as well as the other features discussed above suggest that the anemeta group likely comprises a basal, paraphyletic group within Balcha . Furthermore, although B. splendida always lack setae from the posterior surface of the dorsellum ( Fig. 40 View FIGURES 39–46. 39–42 ), some females have a seta dorsolaterally on the carinate edge that distinguishes the dorsal surface from the posterior surface of the dorsellum. Although in only some females, presence of the seta might indicate that B. splendida is the basal clade of the cylindrica group and is incorrectly included within the laciniosa group from a phylogenetic perspective. Balcha indica is also very similar to B. laciniosa except for the presence of dorsellar setae, which might also indicate that these two species are closely related and further suggests that the defined speciesgroups are not phylogenetically significant.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Balcha Walker
| Gibson, Gary A. P. 2005 |
Sauteria
| Boucek, Z. 1988: 544 |
| Masi, L. 1927: 334 |
Elemba
| Hedqvist, K. - J. 1961: 109 |
| Cameron, P. 1908: 151 |
Balcha
| Walker, F. 1862: 394 |