Nilaparvata lugens (Stal, 1854)
|
publication ID |
https://doi.org/10.1016/j.phytochem.2019.112190 |
|
DOI |
https://doi.org/10.5281/zenodo.8302502 |
|
persistent identifier |
https://treatment.plazi.org/id/F67B0979-FFA6-0F64-FF97-FD3096C6962C |
|
treatment provided by |
Felipe |
|
scientific name |
Nilaparvata lugens |
| status |
|
2.1. OsRIP1 expression is highly induced by N. lugens View in CoL View at ENA infestation
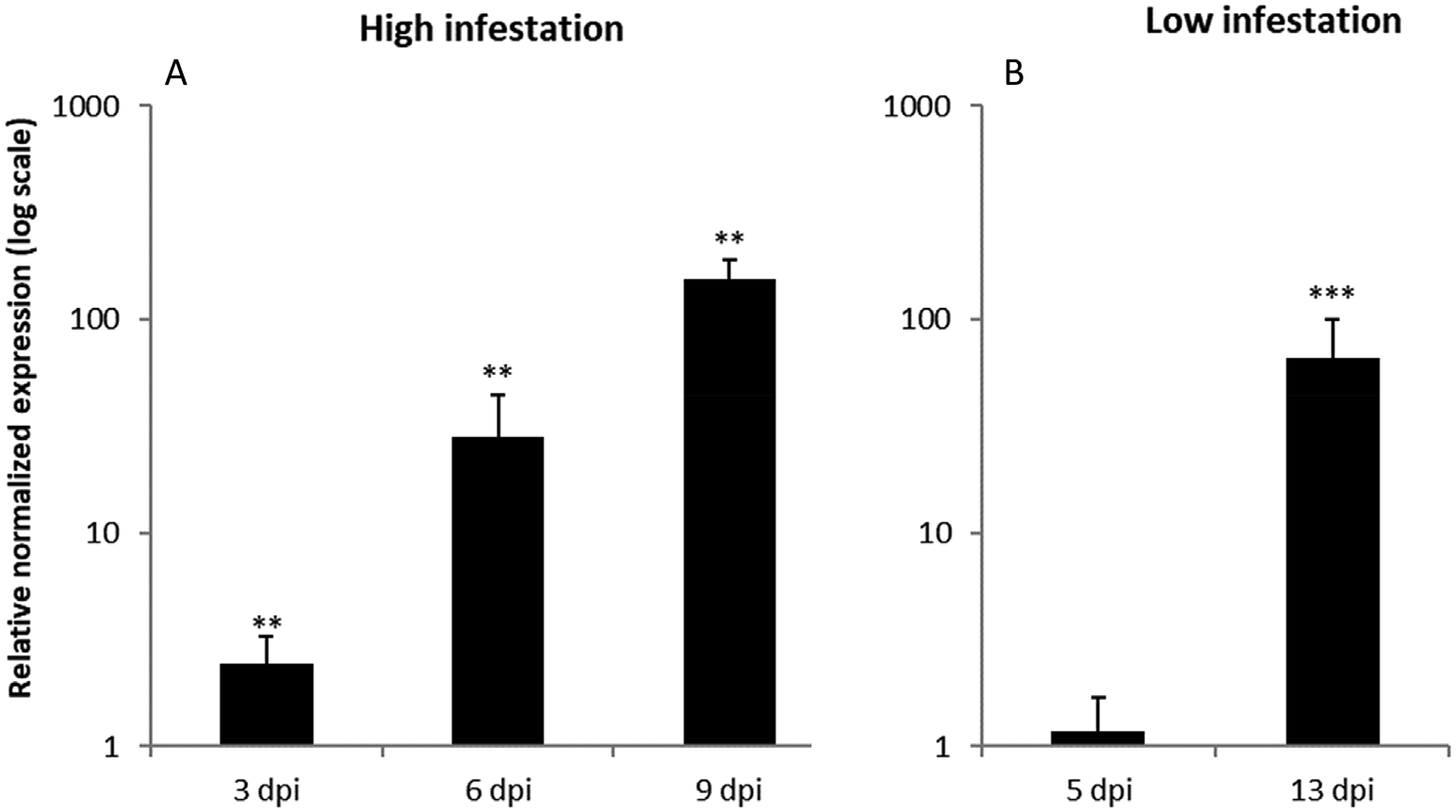
OsRIP1 transcript levels in rice plants exposed to 3rd instar nymphs (nymphal stage N3) of BPH were determined by qPCR analysis. Experiments were performed simulating both high and low infestation conditions. OsRIP1 expression was significantly higher in infested plants compared to control plants at all timepoints analyzed. Transcript levels were more than 100-fold upregulated in infested plants compared to non-infested plants 9 days post infestation (dpi) ( Fig. 1 View Fig ). In the low infestation experiment OsRIP1 expression was not upregulated at 5 dpi. However, at 13 dpi transcript levels were more than 60-fold higher in infested plants compared to non infested control plants ( Fig. 1 View Fig ). These data suggest that OsRIP1 expression in BPH infested rice plants is correlated with the severity of the infestation, with longer infestation periods and higher numbers of BPH increasing OsRIP1 expression.
2.2. Toxic e ff ects of OsRIP1 on BPH
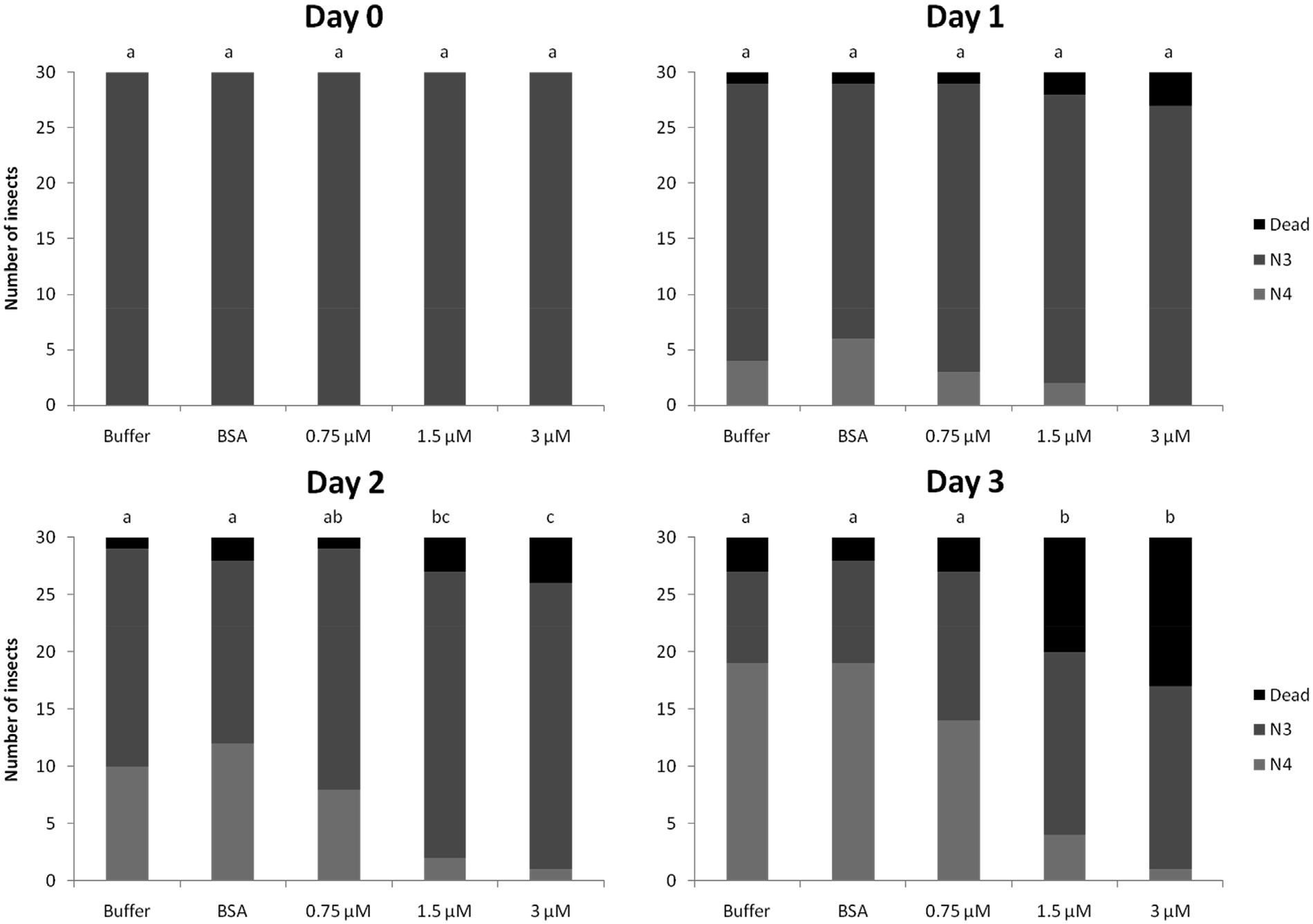
The induction of OsRIP1 expression in rice plants infested by N. lugens suggests a role for OsRIP 1 in plant defense. Therefore, feeding experiments were performed to investigate whether OsRIP1 had a toxic effect on BPH. The artificial diet was supplemented with different concentrations of recombinant OsRIP1 (0.75 μM, 1.5 μM or 3 μM). After two days of feeding, statistically significant dose dependent effects of OsRIP1 on the survival and development of BPH were observed. After three days of feeding, differences between treatments became even more pronounced ( Fig. 2 View Fig ). In the cages exposed to the lowest dose (0.75 μM) of OsRIP1, only 10% of the insects died after three days of feeding. However, when exposed to 1.5 μM or 3 μM of OsRIP1, 33% and 43% of insects died, respectively. In the controls, only 6% and 10% of insects died after three days of feeding with BSA or buffer, respectively. Furthermore, of those insects that survived, 47%, 13% and 3% developed from nymphal stage N3 to nymphal stage N4 when exposed to 0.75 μM, 1.5 μM or 3 μM of OsRIP1, respectively. In both control experiments 65% of the insects developed into N4 stage.
The average biomass of the surviving insects fed with different concentrations of OsRIP1 was significantly lower than the biomass of insects exposed to buffer without protein after three days of feeding ( Fig. 3 View Fig ). No differences in biomass were observed between insects exposed to BSA compared to insects exposed to buffer only ( Fig. 3 View Fig ).
This experimental setup was repeated with slightly older insects (late N3 stage). Since the older insects are more vigorous, the duration of this experiment was prolonged to 5 days. Although more subtle, similar dose dependent effects on survival, development and biomass were observed, confirming the toxic effect of OsRIP1 (see supplementary data, Figures S1 View Fig and S 2 View Fig ).
2.3. OsRIP1 inhibits protein translation of insect ribosomes
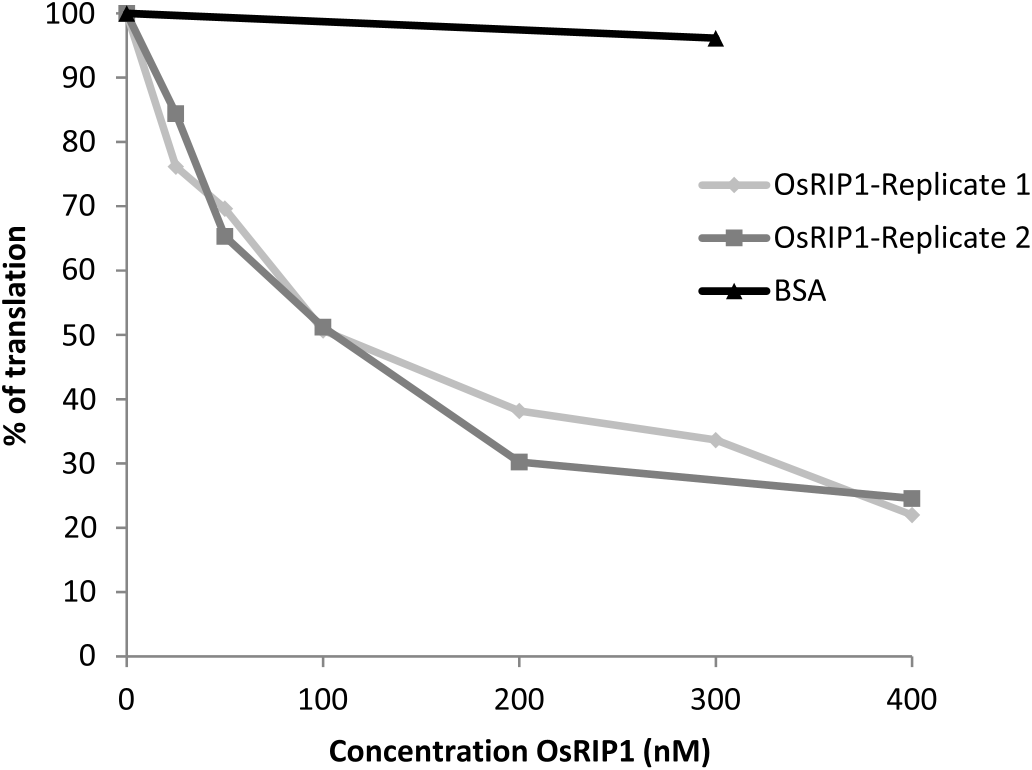
To check whether the observed toxic effects of OsRIP1 towards BPH were the consequence of the enzymatic activity of the protein, the inhibitory effect of recombinant OsRIP1 on translation of insect ribosomes was assessed using a cell-free translation system based on an insect cell extract derived from a S. frugiperda Sf 21 cell line ( Fig. 4 View Fig ). OsRIP1 inhibited protein translation in a dose dependent way while BSA had no effect. The IC50 of OsRIP1 was determined at 100 nM, very similar to the IC 50 in the rabbit reticulocyte system (IC50: 115 nM) but considerably lower than the IC50 for OsRIP 1 in the wheat germ extract system (IC50: 1.5 μM) ( De Zaeytijd et al., 2019).
2.4. E ff ect of OsRIP1 over-expression on insect and plant performance during rice infestation with N. lugens
Oryza sativa (ssp. Japonica cv. Nipponbare) plants over-expressing OsRIP1 under the control of the Ubil promoter were generated. Transgenic lines showed a normal phenotype and seed set (data not shown). OsRIP1 expression was about 24 and 7 times higher in transgenic T 3 shoots from lines J and H, respectively, compared to OsRIP1 expression in wild-type plants ( Figure S3 View Fig ). Two week old wild-type and transgenic plants (T 3 generation) of lines J and H were infested with BPH. Insect survival, development and the biomass of surviving insects were monitored for 10 days. No significant differences in survival, development or biomass were observed between insects reared on transgenic plants or wild-type plants ( Figures S4 View Fig and S5).
After 10 days of infestation, all insects had reached the adult stage. Gender and morphology of the adult BPH were scored. However, no differences were observed between insects reared on wild-type plants and insects on transgenic plants (Figure S6).
To examine the plant performance after N. lugens infestation , the shoot length, shoot biomass and relative water content of wild-type or transgenic mock treated and infested rice plants were determined at the end of the experiment. Infested plants had significantly shorter shoots with a significantly lower biomass than mock treated plants. The relative water content (RWC) of infested plants was significantly lower than the RWC of non-infested plants. No significant differences in shoot length, shoot biomass or RWC were observed between infested wild-type and infested transgenic plants (Figure S7).
3. Discussion
Infestation with BPH resulted in 100-fold enhanced OsRIP1 transcript levels in rice plants, suggesting OsRIP1 plays a role in the defense against N. lugens . In parallel experiments the responsiveness of some stress inducible lectins to BPH infestation was monitored on the same plant samples (data not published). Transcript levels for these lectins only increased 6-30-fold, suggesting that the increase of OsRIP1 transcripts in response to BPH is quite exceptional.
Consequently, the toxic effect of recombinant OsRIP1 against the brown planthopper was examined through feeding assays. A clear dose dependent effect of OsRIP1 on insect survival, development and biomass was observed when compared to insects exposed to buffer control or BSA. When comparing the toxic effects of OsRIP1 against N. lugens with the insecticidal effect of other RIPs, a few things need to be taken into account. First of all, OsRIP1 is a type-1 RIP and type-1 RIPs are generally less toxic than type-2 RIPs because they do not possess a lectin domain promoting their cellular uptake ( Battelli, 2012). However, some type-1 RIPs do show insecticidal activity. For instance, Bertholdo-Vargas et al. (2009) demonstrated the toxic effect of several type-1 RIPs against some Lepidopteran species. A second important aspect to consider when comparing the data for different RIPs is the type of insect that was used in the experiments. Until now, RIP toxicity was mostly investigated for Lepidopteran species ( Zhu et al., 2018). To date, N. lugens was never used in these experiments. However, the toxicity of RIPs for other piercing-sucking insects, in particular Acyrthosiphon pisum , has been determined. SNA-I, a type-2 RIP from Sambucus nigra , and the type-2 RIP from apple ( Malus domestica ), displayed LC50s against A. pisum of 1.56 μM and 500 nM in 3-day feeding experiments, respectively ( Shahidi-Noghabi et al., 2008; Hamshou et al., 2016). The type-1 RIP from apple had an LC50 of 8.8 μM in the same type of experiment. In our feeding experiment with OsRIP1,43% of BPH died when exposed to 3 μM of recombinant OsRIP1 for 3 days. This is a milder insecticidal effect than the type-2 RIPs described above, but compared to the insecticidal effect documented for the type-1 RIP from apple, OsRIP1 has a stronger effect. It is also important to note that the above mentioned experiments with A. pisum were performed with neonate nymphs while this study used older nymphs (early N3 stage). It is expected that toxicity is less severe towards more developed, and therefore more robust, insects. Indeed, when the feeding assay was performed with older planthoppers (late N3 stage), toxic effects of OsRIP1 were more subtle.
It is hypothesized that the observed toxicity towards N. lugens results from the ribosome-inactivating activity of OsRIP1 since recombinantly produced OsRIP1 inhibited protein translation in a cell-free translation system based on insect cell extract, derived from a S. frugiperda sf 21 cell line. The IC50 was 100 nM, similar to the IC50 for OsRIP 1 in a cell free system based on rabbit reticulocyte lysate. Although this activity on S. frugiperda ribosomes does not guarantee that OsRIP1 shows the same activity against N. lugens ribosomes, it serves as a good indication that OsRIP1 is able to inactivate insect ribosomes and most likely exerts its toxicity towards N. lugens through inactivation of ribosomes.
No differences in survival, development and biomass were observed between BPH reared on transgenic rice plants over-expressing OsRIP1 compared to wild-type plants. On plant side, no difference was observed in performance between transgenic and wild-type plants after N. lugens infestation . N. lugens is a piercing-sucking insect that feeds on the phloem sap. OsRIP1 expression in the transgenic plants is under the control of the constitutive Ubil promoter and normally results also in high levels of mRNA in the phloem sap ( Dutt et al., 2012). Most likely, in our experiments OsRIP1 protein levels in the phloem did not amount to sufficient levels to cause inhibitory effects on the insects. In future experiments the use of a strong phloem specific promoter could be used to increase the transgene expression levels in the phloem ( Yin et al., 1997). Phloem fractions could then be collected using the aphid stylectomy technique ( Gaupels et al., 2008) and analyzed for the presence of OsRIP1. Unfortunately, since no antibody against OsRIP1 is presently available, OsRIP1 protein levels in the phloem sap could not be determined and compared to the recombinant protein concentrations tested in the in vitro feeding assays.
The fact that over-expression of OsRIP 1 in transgenic rice plants did not influence the performance of planthoppers reared on these plants, suggests that the present OsRIP1 levels are not capable of protecting the rice plants against the insects. Therefore, the observed up-regulation of OsRIP1 expression in infested wild-type rice plants is most likely not sufficient to protect the plant against N. lugens in a natural environment. It is possible that the observed induction of OsRIP1 gene expression is the result of a more general response to herbivory attack, and not a specific response to brown planthoppers. Therefore, the in vitro and in planta activity of OsRIP1 could be examined for other rice pests and pathogens.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |