Gnesioceros sargassicola ( Mertens, 1833 )
|
publication ID |
https://doi.org/ 10.5852/ejt.2021.736.1249 |
|
publication LSID |
lsid:zoobank.org:pub:FC9085BE-73C4-4F33-BD9B-6A9F573AB01D |
|
DOI |
https://doi.org/10.5281/zenodo.4562014 |
|
persistent identifier |
https://treatment.plazi.org/id/F13387D2-FF80-C12A-FB72-FD05FBA7307D |
|
treatment provided by |
Plazi |
|
scientific name |
Gnesioceros sargassicola ( Mertens, 1833 ) |
| status |
|
Gnesioceros sargassicola ( Mertens, 1833)
Figs 1B View Fig , 7L View Fig
Planaria sargassicola Mertens, 1833: 13–14 , pl. I, figs 4–6.
Stylochus Mertensi Diesing, 1850: 216 View in CoL .
Stylochus sargassicola – Ehrenberg 1836: 67. — Claparède 1861: 143.
Planocera sargassicola – Örsted 1844: 48.
Gnesioceros sargassicola – Diesing 1862: 571.
Gnesioceros Mertensi – Diesing 1862: 572.
Stylochus Mertensi View in CoL – Moseley 1877: 23.
Material examined
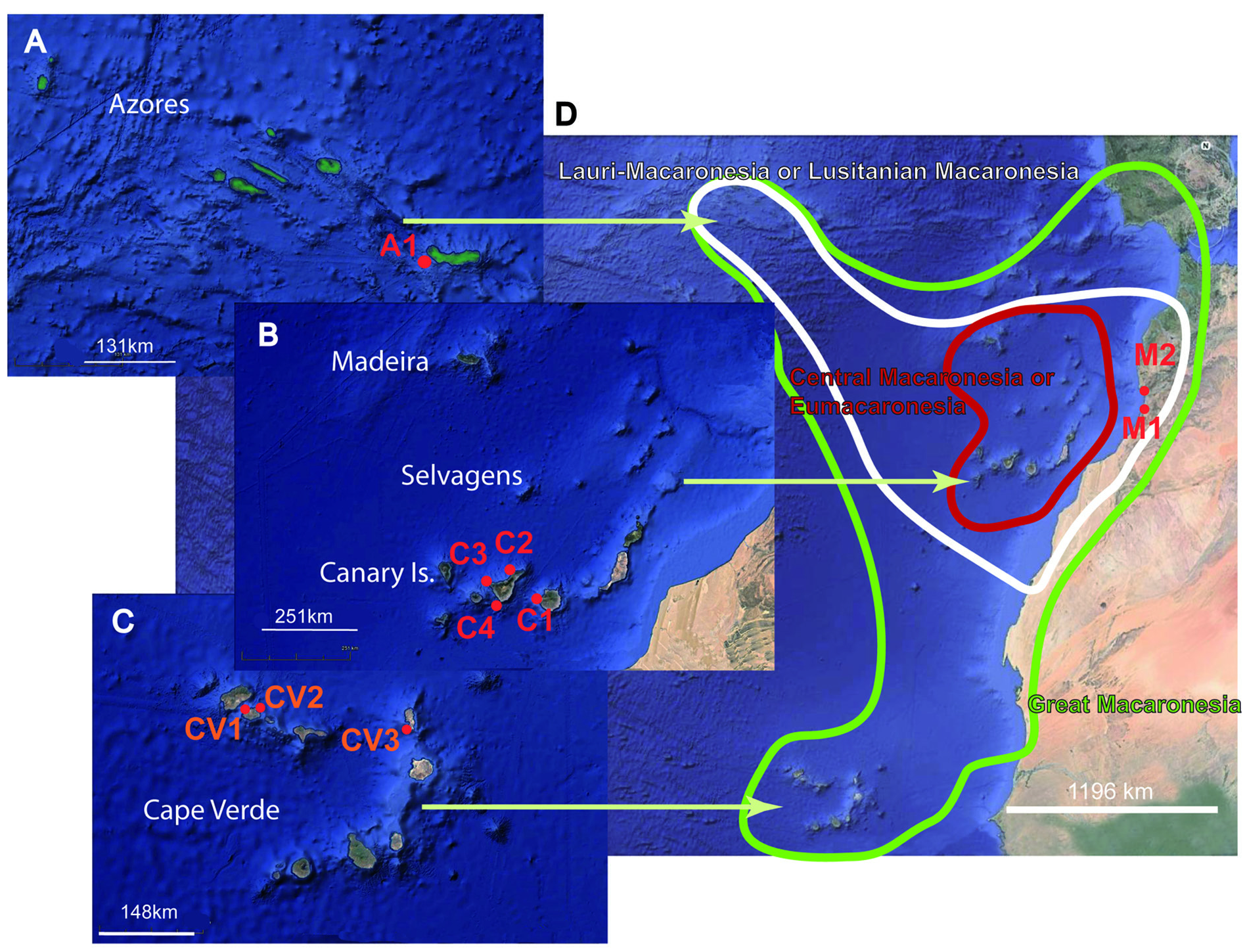
CANARY ISLANDS – Gran Canaria Island • 1 spec.; Pasito Blanco ; 27°44′50.46″ N, 15°37′31.85″ W ( Fig. 1B C1 View Fig ); 4 Jan. 2017; Leopoldo Moro leg.; RCCN. – Tenerife Island GoogleMaps • 1 spec.; 28°24′35.71″ N, 16°18′25.31″ W ( Fig. 1B C View Fig 2 View Fig ); 7 Jun. 2011; Leopoldo Moro leg. RCCN GoogleMaps .
Distribution
Bermuda Islands ( Hyman 1939); Sargasso Sea, Caribbean Sea, Atlantic Ocean ( Faubel 1983); Gulf of Mexico ( Hyman 1954); Atlantic Ocean ( Moseley 1877); Boa Vista Island, Cape Verde ( Laidlaw 1903); Santa Marta, Colombia ( Quiroga 2008); Netherlands, Puerto Rico, Florida, USA, Sargasso Sea, Atlantic Ocean ( Marcus & Marcus 1968).
New records
Pasito Blanco, Gran Canaria, and Tenerife, Canary Islands.
Remarks
Gnesioceros sargassicola was limited to the Antilles and the Caribbean Sea until the record of Laidlaw (1903) for the Cape Verde Islands. The new record of G. sargassicola for the Canary Islands shows a progressive ‘colonisation’ of the Atlantic east coast.
Molecular analysis
The main purpose of the 28S analysis was to confirm the determinations made from the morphological study and verify the relationships among similar species.
The recovered topology by both trees, Bayesian Inference (BI) as well as Maximum Likelihood (ML) strongly supports the monophyly of the suborders Acotylea and Cotylea (Fig. 8; Acotylea : BPP (Bayesian
posterior probabilities) = 1 and BS (Bootstrap values of ML analysis) = 73, Cotylea : BPP = 0.98, BS = 100).
Within Acotylea , the monophyly of Leptoplanoidea (BPP = 1, BS = 97) and Stylochoidea (BPP = 1, BS = 73) is well supported. Callioplana marginata Stimpson, 1857 considered within the superfamily Stylochoidea appears isolated with the highest support (BS = 100).
As a sister group of Leptoplanoidea , there is a clade of species: Ilyella gigas ( Schmarda 1859) , Discocelis tigrina ( Blanchard, 1847) , Adenoplana evelinae Marcus, 1950 , Amemiyaia pacifica Kato, 1944 and Phaenocelis medvedica Marcus, 1952 showing low support and not clearly grouped (Fig. 8).
The main group of Leptoplanoidea encloses the family Leptoplanidae , with the genera Leptoplana Ehrenberg, 1831 and Armatoplana Faubel, 1983 , and the family Notoplanidae Marcus & Marcus, 1966 with the genera Notoplana Laidlaw, 1903 and Notocomplana Faubel, 1983 . Pseudostylochus Yeri & Kaburaki, 1918 and Koinostylochus Faubel, 1983 appear together and belong to the family Pseudostylochidae Faubel, 1983 .
Within Stylochoidea , four main clades are recovered: family Stylochidae with the genera Stylochus , Imogine Girard, 1853 and Paraplanocera Laidlaw, 1903 (see the Discussion); family Latocestidae Laidlaw, 1903 with Leptostylochus Bock, 1925 and Latocestus ; family Hoploplanidae StummerTraunfels, 1933 with Hoploplana Laidlaw, 1902 and finally family Planoceridae with Paraplanocera Laidlaw, 1903 and Planocera . Callioplana marginata can be considered a sister group of Stylochoidea .
Within the suborder Cotylea , the families Cestoplanidae Lang, 1884 , Pericelidae Laidlaw, 1902 and Anonymidae represented by the genera Cestoplana Lang, 1884 (BPP = 1, BS 100), Pericelis (BPP = 1, BS 100) and Anonymus (BPP = 1, BS 100), respectively are presented as isolated groups. While Boninia Bock, 1923 , Chromyella Correa, 1958 and Theama Marcus, 1949 form a well-supported group (BPP = 1, BS = 100), although currently belonging to separate families and not specifically related to each other.
After Anonymidae , we find a well-supported branch (BPP = 0.9, BS = 95) that encompasses the families Prosthiostomidae Lang, 1884 , Euryleptidae and Pseudocerotidae . This branch is, in turn, divided into two main branches (both supported by maximum values: BPP = 1, BS = 100) where Prosthiostomidae is separated from Euryleptidae and Pseudocerotidae , families with a clear relation.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
SubPhylum |
Rhabditophora |
|
Class |
|
|
Order |
|
|
SubOrder |
Acotylea |
|
SuperFamily |
Leptoplanoidea |
|
Family |
|
|
Genus |
Gnesioceros sargassicola ( Mertens, 1833 )
| Cuadrado, Daniel, Rodríguez, Jorge, Moro, Leopoldo, Grande, Cristina & Noreña, Carolina 2021 |
Stylochus Mertensi
| Moseley H. N. 1877: 23 |
Gnesioceros sargassicola
| Diesing C. M. 1862: 571 |
Gnesioceros
| Diesing C. M. 1862: 572 |
Stylochus Mertensi Diesing, 1850: 216
| Diesing C. M. 1850: 216 |
Planocera sargassicola
| Örsted 1844: 48 |
Stylochus sargassicola
| Claparede E. 1861: 143 |
| Ehrenberg C. G. 1836: 67 |
Planaria sargassicola
| Mertens H. 1833: 14 |