Milnesium quiranae, Rocha & González-Reyes & Ostertag & Lisi, 2022
|
publication ID |
https://doi.org/10.5852/ejt.2022.822.1807 |
|
publication LSID |
lsid:zoobank.org:pub:522FD009-B4C9-4A80-871E-883E2EBE09C8 |
|
persistent identifier |
https://treatment.plazi.org/id/E27887D6-CA42-FFD0-FDF5-FDF7FDADFC5A |
|
treatment provided by |
Felipe |
|
scientific name |
Milnesium quiranae |
| status |
sp. nov. |
Milnesium quiranae sp. nov.
urn:lsid:zoobank.org:act:DDC4D209-B7E0-411A-8D83-0E086CB44D04
Figs 19–21 View Fig View Fig View Fig , Tables 8–11
Diagnosis
Smooth cuticle, six peribuccal lamellae, six peribuccal papillae with the medioventral reducing with growing, two lateral cephalic papillae. Buccal tube nearly cylindrical in all life stages, becoming wider with growing. Claws with configuration [3-3]-[3-3] in all life stages.
Etymology
The new species is dedicated to the researcher Estela Maris Quirán, whopublished important contributions on the scientific knowledge of invertebrates in La Pampa, Argentina.
Material examined
Holotype ARGENTINA • ♀; Salta Province, Salta City; 24°47′18″ S, 65°24′38″ W; 1150 m a.s.l.; 2 May 2014; Rocha-Doma leg.; moss and lichens from trees; MCNS Tar.000024(3) . GoogleMaps
Paratypes ARGENTINA • 2 ♀♀; same collection data as for holotype; MCNS Tar.000025(3) , Tar.000025(4) GoogleMaps • 4 ♀♀; same collection data as for holotype; UNICT 5900(1) to 5900(4) GoogleMaps • 21 ♀♀; same collection data as for holotype; UNLPam 558(1) to 558(3) , 558(5) , 560(1) , 560(3) , 560(4) , 564(4) , 565(1) , 565(4) , 565(5) , 566(1) to 566(4) , 570(1) , 573(5) , 576(4) , 576(5) , 586(1) , 586(2) GoogleMaps .
Morphological description
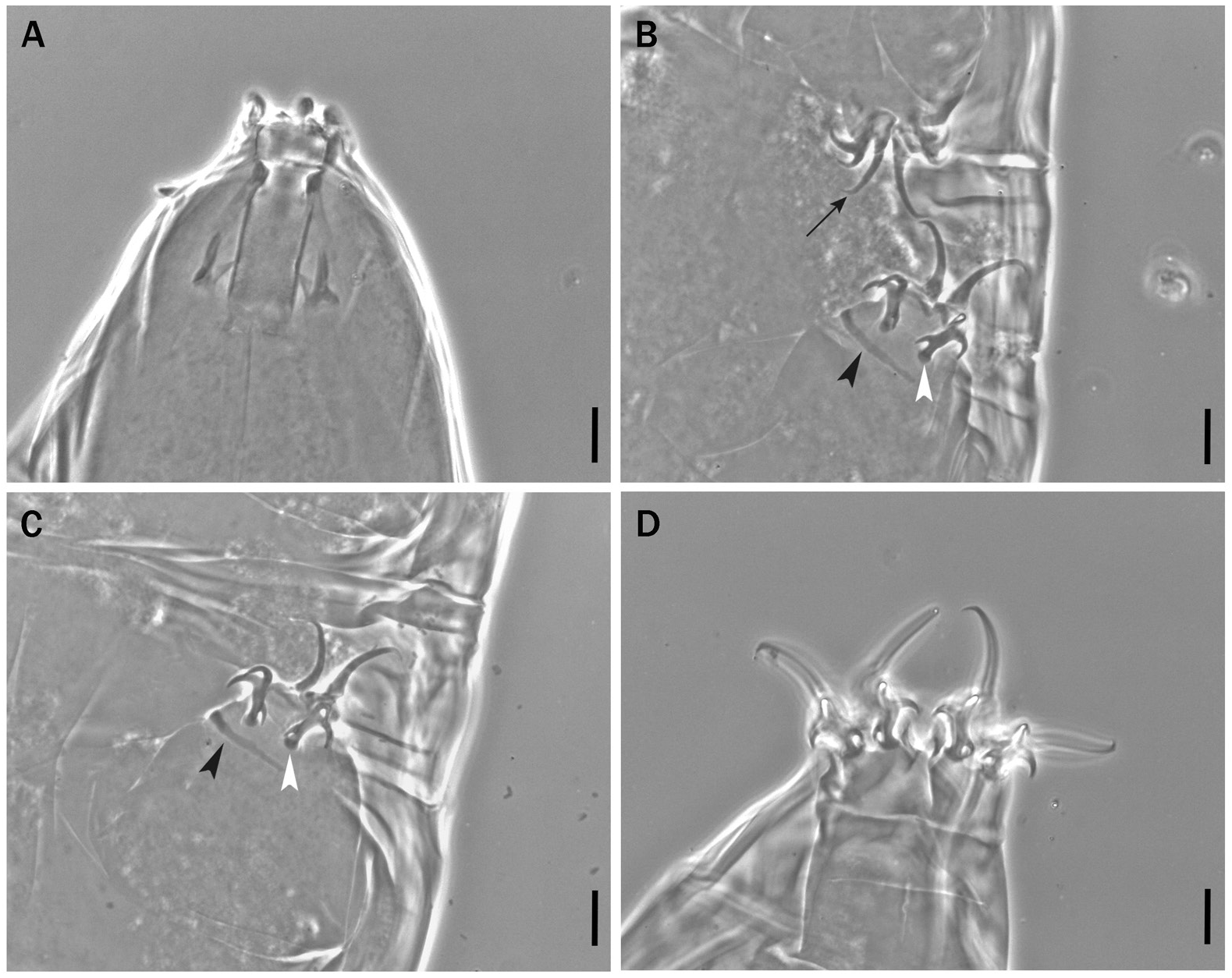
Body length up to 770 µm (habitus in Fig. 20A View Fig ), reddish colour before mounting, eyes present. Cuticle smooth ( Fig. 20B–C View Fig ) without dimples, wrinkles, pseudopores, reticulations, pseudoplates or gibbosities. Six peribuccal lamellae and six peribuccal plus two lateral papillae present; medio-ventral peribuccal papilla reduced in senior specimens ( Fig. 21B View Fig black arrowhead). Buccal tube ( Figs 19A View Fig , 21A View Fig ) from slightly funnel-shaped to almost cylindrical (posterior/anterior width ratio 80–98%), wider in senior specimens; pt of stylet support insertion point on the buccal tube [65.5–75.1]. Stylet furcae relatively large ( Figs 19A View Fig , 21A View Fig ).
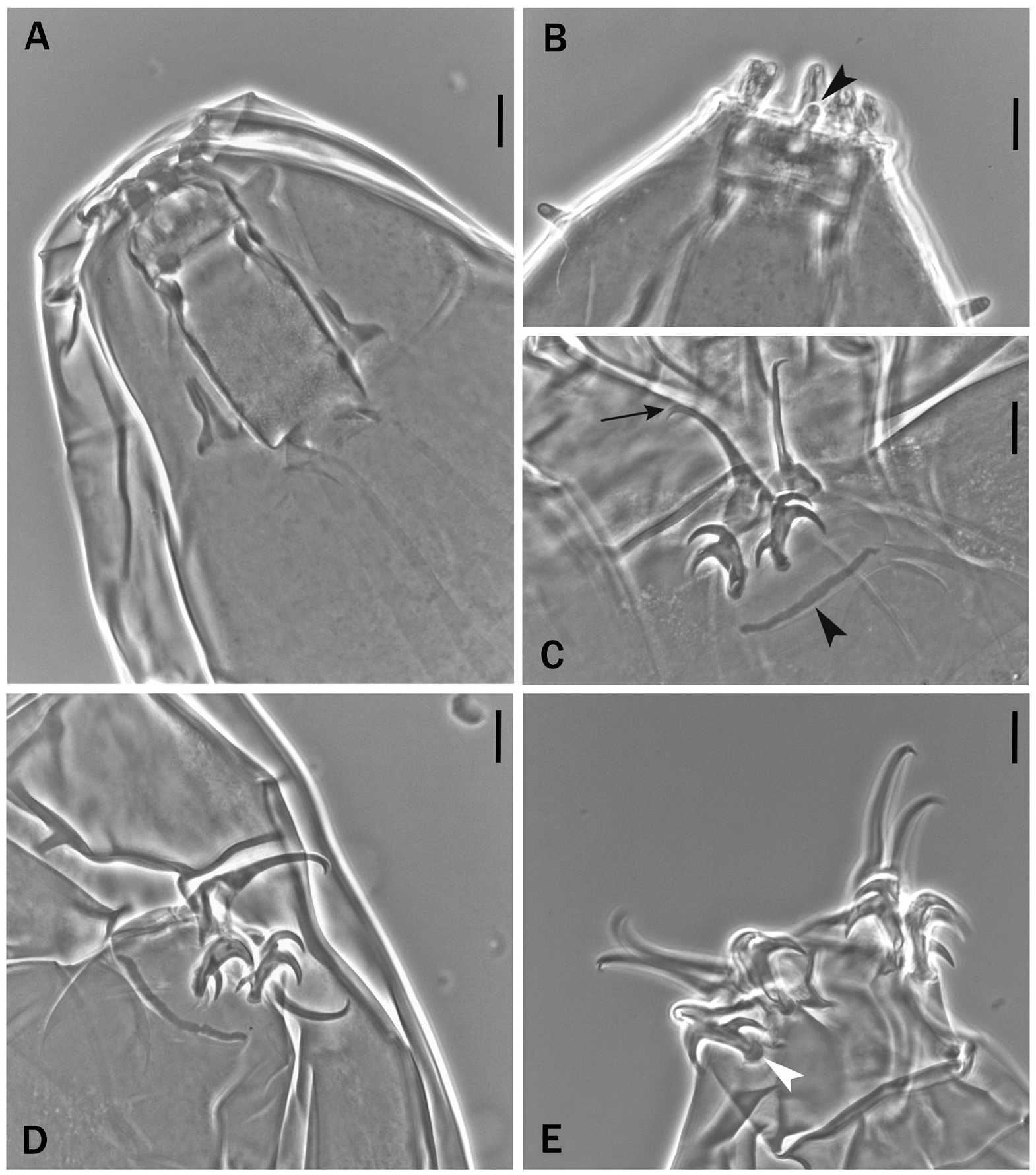
Claws ( Figs 19B–D View Fig , 21C–E View Fig ) of the Milnesium type with configuration [3-3]-[3-3]. Secondary branches of legs IV more robust than on legs I–III (this difference is less marked in senior specimens; compare Fig. 19B–C View Fig with 19D, and Fig. 21C–D View Fig with 21E); basal spurs of internal claws of legs I–III and anterior claws of legs IV more developed than external I–III and posterior IV ( Tables 8 and 9), but this difference may not be visible depending on claw position. Secondary branches with basal thickenings (‘lunulae’, Figs 19B–C View Fig , 21E View Fig , white arrowheads), primary branches with very small accessory points ( Figs 19B View Fig , 21C View Fig , black arrows); cuticular bars present on legs I–III ( Figs 19C View Fig , 21C View Fig , black arrowheads). Percentual ratio of secondary branches with respect to primary branches for each couple slightly higher for legs I than for all other legs (for all specimens collectively, legs I have 66–95% vs legs II–IV 62–86%; Tables 8–10); the ratio for all legs is on average slightly lower in senior than in young specimens (for all legs collectively, young specimens have 66–95% vs 63–87% in senior; compare Table 8 with Table 9).
Young specimens (hatchlings or hatchlings plus second instar: 270–460 µm; Fig. 19 View Fig , Table 8, Supp. file 4)
Medioventral peribuccal papilla similar in size to the others; buccal tube ( Fig. 19A View Fig ) more slender (pt of buccal tube standard width [43.2–51.5]).
Intermediate specimens (probably second or third instar: 476–568 µm; Table 10, Supp. file 6) These specimens show intermediate metric characters between young and senior.
Senior specimens (probably from third or fourth instar on: 585–770 µm; Figs 20–21 View Fig View Fig , Table 9, Supp. file 5)
Medio-ventral peribuccal papilla reduced ( Fig. 21B View Fig black arrowhead; pt of such papilla [9.8–12.5] vs [22.6–31.6] of the other peribuccal papillae); buccal tube wider (pt of buccal tube standard width [59.1–67.9]; Fig. 21A View Fig ).
Remarks
The medioventral peribuccal papilla is reduced in senior specimens (comments in Discussion). Morphometric data are given in Table 8 for young, Table 9 for senior, Table 10 for intermediate specimens; in Table 11 the statistically significant differences (through Student t -test) of overlapping pt ranges of claw heights between the new species and the similar ones.
Differential diagnosis
Based on having smooth cuticle, M. quiranae sp. nov. belongs to the old tardigradum group ( Michalczyk et al. 2012a, 2012b). The new species, lacking pseudopores and pseudoplates, and having three points on the secondary branches of all claws [3-3]-[3-3] and six peribuccal lamellae, is similar to M. antarcticum Tumanov, 2006 ; M. asiaticum Tumanov, 2006 ; M. barbadosense Meyer & Hinton, 2012 ; M. bohleberi Bartels, Nelson, Kaczmarek & Michalczyk, 2014 ; M. brachyungue Binda & Pilato, 1990 ; M. burgessi Schlabach, Donaldson, Hobelman, Miller & Lowman, 2018 ; M. eurystomum Maucci, 1991 (emended by Morek et al. 2020a); M. longiungue Tumanov, 2006 ; M. minutum Pilato & Lisi, 2016 ; M. pseudotardigradum Surmacz, Morek & Michalczyk, 2019 M. sandrae Pilato & Lisi, 2016 ; M. shilohae Meyer, 2015 ; M. swansoni Young, Chappell, Miller & Lowman, 2016 ; M. tumanovi Pilato, Sabella & Lisi, 2016 ; M. validum Pilato, Sabella, D’Urso & Lisi, 2017 ; M. zsalakoae Meyer & Hinton, 2010 .
For more precise comparisons, we first compare only senior specimens of the new species with all mentioned similar species, apart from M. minutum , which was very probably described based on young specimens and is therefore compared with the young of M. quiranae sp. nov.; senior specimens of M. quiranae differ from:
1. Milnesium antarcticum , only known from the type locality in Antarctica, by different body colour: reddish in M. quiranae sp. nov. vs reddish-brown in M. antarcticum ; different buccal tube width: higher pt of standard width [59.1–67.9] in M. quiranae vs [35.4–43.9] in M. antarcticum ; different pt of many claw heights: external primary and secondary branches I [45.6–54.9] and [34.2–41.8] in M. quiranae vs [34.0–43.2] and [22.9–28.4] respectively in M. antarcticum ; external primary and secondary branches III [49.3–58.3] and [36.2–43.1] in M. quiranae vs [38.5–45.7] and [18.9–21.1] respectively in M. antarcticum ; posterior primary and secondary branches IV [60.1–69.7] and [44.0– 51.7] in M. quiranae vs [49.8–55-9] and [28.0–34.3] respectively in M. antarcticum .
2. Milnesium asiaticum only known from the type locality in Kyrgyz Republic (Central Asia), by different body colour: reddish in M. quiranae sp. nov. vs slightly reddish or white in M. asiaticum ; higher pt of buccal tube standard width [59.1–67.9] in M. quiranae vs [30.0–41.6] in M. asiaticum ; higher pt of stylet supports insertion point [67.5–73.6] in M. quiranae vs [63.9–66.9] in M. asiaticum . Statistically significant differences about pt of several claw heights ( Table 11).
3. Milnesium barbadosense known from the type locality in Barbados Islands (Caribbean Sea) and from Mexico ( Moreno-Talamantes et al. 2019, 2020); by different body colour: reddish in M. quiranae sp. nov. vs white or transparent in M. barbadosense ; eyes present in M. quiranae vs absent in M. barbadosense ; different buccal tube width: higher pt of standard width [59.1–67.9] in M. quiranae vs [27.2–49.7] in M. barbadosense ; statistically significant differences about pt of many claw heights ( Table 11).
4. Milnesium bohleberi , only known from the type locality in USA, by different body colour: reddish in M. quiranae sp. nov. vs white or transparent in M. bohleberi ; eyes present in M. quiranae vs absent in M. bohleberi ; statistically significant higher pt of the peribuccal papillae [22.6–31.6, mean 25.8] in M. quiranae vs [27.2–32.3, mean 30.1] in M. bohleberi (t7 = -4.42, p <0.005); statistically significant differences about pt of buccal tube anterior width [57.9–66.1, mean 62.5] in M. quiranae vs [63.4– 74.7, mean 68.1] in M. bohleberi (t 15 = -5.12, p <0.001); statistically significant differences about pt of several claw heights ( Table 11).
5. Milnesium brachyungue , known from Chile (type locality) and Colombia ( Londoño et al. 2015); by different body colour: reddish in M. quiranae sp. nov. vs transparent in M. brachyungue ; different buccal tube width: higher pt of standard width [59.1–67.9] in M. quiranae vs [37.0] in M. brachyungue ; different pt of many claw heights: external primary and secondary branch I [45.6–54.9] and [34.2–41.8] in M. quiranae vs [22.8] and [22.8] respectively in M. brachyungue ; external primary and secondary branch II [47.6–56.6] and [36.4–42.5] in M. quiranae vs [24.5] and [23.9] respectively in M. brachyungue ; external primary and secondary branch III [49.3–58.3] and [36.2–43.1] in M. quiranae vs [27.1] and [23.7] respectively in M. brachyungue ; posterior primary and secondary branch IV [60.1–69.7] and [44.0–51.7] in M. quiranae vs [33.1] and [24.6] respectively in M. brachyungue .
6. Milnesium burgessi only known from the type locality in USA; by different body colour: reddish in M. quiranae sp. nov. vs transparent to yellow in M. burgessi ; pseudoplates absent in the new species vs present in M. burgessi ; statistically significant differences of pt of all branches I–IV in M. quiranae ( Table 11) including anterior primary branch IV [56.4–65.6] in M. quiranae vs [70.7–89–6] in M. burgessi (statistical significance not calculated due to completely separate ranges).
7. Milnesium eurystomum known from Greenland (type locality), from Argentina and Chile ( Maucci 1996), Mongolia ( Kaczmarek & Michalczyk 2006), Arkansas, USA ( Land et al. 2012), Alaska, USA ( Johansson et al. 2013), Norway, United Kindong ( Scotland) ( Morek et al. 2020a), by different body colour: reddish in M. quiranae vs brownish in M. eurystomum ; no evident ontogenetic shape change of the buccal tube in M. quiranae (which remains more or less cylindrical) vs marked ontogenetic shape change in M. eurystomum (the tube becomes definitely funnel-shaped); statistically significant higher buccal tube posterior/anterior width ratio, 80–97%, mean 92% in M. quiranae vs 53–92%, mean 75% in M. eurystomum (t 15 = 4.72, p <0.001); statistically significant higher pt of stylet supports insertion point [67.5–73.6, mean 70.5] in M. quiranae vs [60.3–69.8, mean 65.1] in M. eurystomum (t 15 = 4.51, p <0.001); different pt of external primary branch I and III [45.6–54.9] and [49.3–58.3] in M. quiranae vs [30.3–43.3] and [31.8–48.1] respectively in M. eurystomum ; internal primary branch II [46.3–54.9] in M. quiranae vs [30.5–45.6] in M. eurystomum posterior primary branch IV [60.1–69.7] in M. quiranae vs [35.4–58.9] in M. eurystomum ; statistically significant differences of pt of many other claw heights ( Table 11).
8. Milnesium longiungue , only known from the type locality in India, by different body colour: reddish in M. quiranae sp. nov. vs white in M. longiungue ; different buccal tube width: higher pt of standard width [59.1–67.9] in M. quiranae vs [33.8–59.1] in M. longiungue ; accessory points present in M. quiranae vs absent in M. longiungue ; higher pt of the stylet support insertion point: [67.5–73.6] in M. quiranae vs [59.1–66.7] in M. longiungue ; statistically significant differences about pt of external primary branch III ( Table 11); lower pt of posterior primary branch IV [60.1–69.7] in M. quiranae vs [81.8–92.4] in M. longiungue .
9. Milnesium pseudotardigradum , only known from the type locality in Iceland, by different body colour: reddish in M. quiranae sp. nov. vs yellowish in M. pseudotardigradum ; statistically significant higher pt of the peribuccal papillae [22.6–31.6, mean 25.8] in M. quiranae vs [13.7–24.8, mean 19.9] in M. pseudotardigradum (t7 = 2.56, p <0.05); statistically significant higher pt of the lateral papillae [18.8–27.2, mean 21.5] in M. quiranae vs [11.0–21.8, mean 15.6] in M. pseudotardigradum ; t15 = 3.83, p <0.001; different buccal tube width: pt of standard width [59.1–67.9] in M. quiranae vs [25.5–50.8] in M. pseudotardigradum ; claw configuration: [3-3]-[3-3] in all the specimens (young and senior specimens) in M. quiranae vs [3-3]-[3-3] only in hatchlings in M. pseudotardigradum ; changes during the ontogeny of claw configuration are absent in M. quiranae vs with double change in M. pseudotardigradum .
10. Milnesium sandrae , only known from the type locality in Hawaii ( USA), by different body colour, reddish in M. quiranae vs transparent in M. sandrae ; different buccal tube width: pt of standard width [59.1–67.9] in M. quiranae vs [44.9–48.0] in M. sandrae ; pt of the stylet support insertion point: [67.5– 73.6] in M. quiranae vs [58.0–60.5] in M. sandrae ; by different pt of many claw heights: external primary branches I–III [45.6–54.9]-[47.2–56.6]-[49.3–58.3] in M. quiranae vs [38.8–43.5]-[42.4–46.6]- [43.4–46.1] respectively in M. sandrae ; posterior primary and secondary branch IV [60.1–69.7] and [44.0–51.7] in M. quiranae vs [54.0–57.1] and [38.0–40.2] respectively in M. sandrae , and statistically significant differences of pt of external secondary branches I–III ( Table 11).
11. Milnesium shilohae , only known from the type locality in Hawaii ( USA), by different body colour: reddish in M. quiranae sp. nov. vs white or transparent in M. shilohae ; statistically significant difference of pt of lateral papillae [18.8–27.2, mean 21.5] in M. quiranae vs [12.8–21.8, mean 17.2] in M. shilohae (t 15 = 3.73, p <0.001); different buccal tube width: lower pt of standard width, [59.1–67.9] in M. quiranae vs [47.1–55.9] in M. shilohae ; lower pt of stylet support insertion point: [67.5–73.6] in M. quiranae vs [75.5–77.5] in M. shilohae ; different pt of many claw heights: external primary branch I [45.6–54.9] in M. quiranae vs [34.2–40.3] in M. shilohae , external primary and secondary branches II [47.2–56.6] and [36.4–42.5] in M. quiranae vs [37.4–44.1] and [28.2–35.9] respectively in M. shilohae ; internal primary and secondary branches II [46.3–54.9] and [35.5–40.2] in M. quiranae vs [35.7–42.0] and [28.8–34.5] respectively in M. shilohae ; external primary branch III [49.3–58.3] in M. quiranae vs [35.2–46.8] in M. shilahoe ; statistically significant differences of pt of external secondary branch III ( Table 11); different pt anterior and posterior primary branches IV [56.4–65.6] and [60.1–69.7] in M. quiranae vs [42.6–51.1] and [48.3–55.5] respectively in M. shilohae and statistically significant differences about pt of external secondary branch I, external secondary branch III and anterior and posterior secondary branches IV ( Table 11).
12. Milnesium swansoni only known from the type locality in USA; by different body colour: reddish in M. quiranae sp. nov. vs transparent to yellow in M. swansoni ; higher pt of buccal tube standard width [59.1–67.9] in M. quiranae vs [39.2–42.2] in M. swansoni , statistically significant higher pt of the stylet support insertion point: [67.5–73.6] in M. quiranae vs [66.6–68.2] in M. swansoni (t 15 = 7.39, p <0.001).
13. Milnesium tumanovi only known from the type locality in Yalta (Crimea) by body colour: reddish in M. quiranae sp. nov. vs transparent in M. tumanovi ; different buccal tube width: higher pt of standard width [59.1–67.9], in M. quiranae vs [55.1] in M. tumanovi ; higher pt of the stylet support insertion point: [67.5–73.6] in M. quiranae vs [52.3] in M. tumanovi ; different pt of the external primary branches I–III [45.6–58.3], [47.2–56.6] and [49.3–58.3] respectively in M. quiranae vs [43.0], [43.4] and [32.6] in M. tumanovi , external secondary branches I and III [34.2–41.8], [36.2–43.1] respectively in M. quiranae vs [32.6] and [33.0] respectively in M. tumanovi , posterior primary and secondary branch IV [60.1–69.7] and [44.0–51.7] in M. quiranae vs [55.3] and [42.2] respectively in M. tumanovi .
14. Milnesium validum , only known from the type locality in Antarctica, by different body colour, reddish in M. quiranae sp. nov. vs colourless in M. validum ; different buccal tube width: higher pt of standard width [59.1–67.9] in M. quiranae vs [29.9–43.9] in M. validum ; higher pt of stylet support insertion point: [67.5–73.6] in M. quiranae vs [62.0–65.1] in M. validum ; higher pt of several claw heights: external primary and secondary branches I [45.6–54.9] and [34.2–41.8] in M. quiranae vs [36.0–38.3] and [25.4–28.6] respectively in M. validum ; external primary and secondary branches II [47.2.-56.6] and [36.4–42.5] in M. quiranae vs [37.2–42.1] and [27.0–30.3] respectively in M. validum ; external primary and secondary branches III [49.4–58.3] and [36.2–43.1] in M. quiranae vs [38.5–42.5] and [30.4] respectively in M. validum , and posterior primary and secondary branches IV [60.1–69.7] and [41.4–49.6] in M. quiranae vs [47.9–49.3] and [28.8–32.9] respectively in M. validum .
15. Milnesium zsalakoae only known from the type locality in USA by different body colour: reddish in M. quiranae sp. nov. vs white or transparent in M. zsalakoae ; different buccal tube width: pt of standard width [59.1–67.9] in M. quiranae vs [36.8–41.9] in M. zsalakoae ; accessory points present in M. quiranae vs absent in M. zsalakoae ; different pt of several claw heights: internal primary branches I–III [43.2– 52.0], [46.3–54.9], [46.0–56.0] in M. quiranae . vs [64.4–68.6], [64.7–80.4], [80.5–88.6], respectively in M. zsalakoae ; internal secondary branch I [30.6–38.9] in M. quiranae vs [45.4–64.7] in M. zsalakoae and anterior primary branch IV [56.4–65.6] in M. quiranae vs [94.8–102.9] in M. zsalakoae .
With regard to young and intermediate specimens of M. quiranae sp. nov., as already indicated, differences of senior specimens with the above mentioned species tend to remain valid, because the only ontogenetic changes in the new species pertain to the buccal tube width and the medioventral peribuccal papilla reduction; thus, apart from those two characters, the already provided differential diagnosis is valid also for young and intermediate specimens of M. quiranae (e.g., considering body colour, presence/absence of eyes, pt of stylet support insertion point and of claw heights). It is only worth mentioning that for M. burgessi , M. longiungue , M. pseudotardigradum , M. sandrae , M. shilohae and M. tumanovi , the buccal tube width is comparable with with young and/or intermediate specimens of M. quiranae , but in all cases there are the other differences that keep the species well differentiated. We here provide detailed comparison of young specimens of M. quiranae only with M. minutum , for the reasons expressed above.
Young specimens of M. quiranae sp. nov. differ from M. minutum (only known from the type locality in Italy) by different body colour: reddish in M. quiranae sp. nov. vs transparent in M. minutum ; different buccal tube width: higher pt of buccal tube standard width [43.2–51.5] in M. quiranae vs [38.6–42.4] in M. minutum ; higher pt of the stylet support insertion point: [67.4–75.1] in M. quiranae vs [63.0–65.9] in M. minutum ; different pt of many claw heights: external primary and secondary branches I, [39.8–49.8] and [31.2–40.6] in M. quiranae vs [39.1] and [28.3] in M. minutum ; external primary and secondary branches II [45.0–54.5] and [33.5–43.3] in M. quiranae vs [42.2–44.3] and [29.5–31.4] respectively in M. minutum ; posterior secondary branches IV [37.4–47.2] in M. quiranae vs [33.5–34.5] in M. minutum .
Dichotomous key of the species of Milnesium Doyère, 1840 from South America
1. Dorsal cuticle smooth ....................................................................................................................... 2
– Dorsal cuticle ornamented (pseudopores-reticulum-dimples) .......................................................... 5
2. Claw configuration [2-2]-[2-2] .......................................................... M. kogui Londoño et al., 2015
– Claw configuration [3-3]-[3-3] ......................................................................................................... 3
3. BT funnel-shaped in adults (posterior/anterior width ratio lower than 65%) ..................................... .............................................. M. eurystomum Maucci, 1991 (amended by Michalczyk et al. 2012b; amended by Morek et al. 2020a)
– BT about cylindrical in adults (posterior/anterior width ratio higher than 75%) ............................. 4
4. Pt of BT standard width lower than 52 in adults .................. M. brachyungue Binda & Pilato, 1990
– Pt of BT standard width higher than 52 in adults .............................................. M. quiranae sp. nov.
5. Claw configuration [3-3]-[3-3] ......................................................................................................... 6
– Claw configuration different ............................................................................................................. 7
6. Nine rows of pseudoplates, pt of SS [63.8–66.7]; pt of BT standard width [58.1–65.6] ................... ..................................................................................................... M. beatae Roszkowska et al., 2015
– Without pseudoplates, pt of SS [70.0–73.7], pt of BT standard width [24.2–32.3] ........................... ............................................................................................ M. argentinum Roszkowska et al., 2015
7. Claw configuration [2-2]-[2-2] .............................................. M. katarzynae Kaczmarek et al., 2004
– Claw configuration different ............................................................................................................. 8
8. Claw configuration [2-3]-[2-2], pt of BT standard width [46.1–56.9], with 9 rows of pseudoplates ... ................................................................................................................................... M. irenae sp. nov.
– Claw configuration [2-3]-[3-2] .............................................................................................................9
9. Cuticular dimples arranged in 10 bands, small tubercles around all claw bases, pt of BT standard width [55.2–64.0], young specimens with claw configuration [2-2]-[2-2] .... M. pelufforum sp. nov.
– Cuticular dimples present not forming bands, pt of BT standard width [33.0–38.0], no tubercles around claw bases ..................................................... M. krzysztofi Kaczmarek & Michalczyk, 2007
Abbreviations: BT = buccal tube; SS = stylet support insertion point on the buccal tube.
Dichotomous key of the species of Milnesium Doyère, 1840 from South America
1. Dorsal cuticle smooth ....................................................................................................................... 2
– Dorsal cuticle ornamented (pseudopores-reticulum-dimples) .......................................................... 5
2. Claw configuration [2-2]-[2-2] .......................................................... M. kogui Londoño et al., 2015
– Claw configuration [3-3]-[3-3] ......................................................................................................... 3
3. BT funnel-shaped in adults (posterior/anterior width ratio lower than 65%) ..................................... .............................................. M. eurystomum Maucci, 1991 (amended by Michalczyk et al. 2012b; amended by Morek et al. 2020a)
– BT about cylindrical in adults (posterior/anterior width ratio higher than 75%) ............................. 4
4. Pt of BT standard width lower than 52 in adults .................. M. brachyungue Binda & Pilato, 1990
– Pt of BT standard width higher than 52 in adults .............................................. M. quiranae sp. nov.
5. Claw configuration [3-3]-[3-3] ......................................................................................................... 6
– Claw configuration different ............................................................................................................. 7
6. Nine rows of pseudoplates, pt of SS [63.8–66.7]; pt of BT standard width [58.1–65.6] ................... ..................................................................................................... M. beatae Roszkowska et al., 2015
– Without pseudoplates, pt of SS [70.0–73.7], pt of BT standard width [24.2–32.3] ........................... ............................................................................................ M. argentinum Roszkowska et al., 2015
7. Claw configuration [2-2]-[2-2] .............................................. M. katarzynae Kaczmarek et al., 2004
– Claw configuration different ............................................................................................................. 8
8. Claw configuration [2-3]-[2-2], pt of BT standard width [46.1–56.9], with 9 rows of pseudoplates ... ................................................................................................................................... M. irenae sp. nov.
– Claw configuration [2-3]-[3-2] .............................................................................................................9
9. Cuticular dimples arranged in 10 bands, small tubercles around all claw bases, pt of BT standard width [55.2–64.0], young specimens with claw configuration [2-2]-[2-2] .... M. pelufforum sp. nov.
– Cuticular dimples present not forming bands, pt of BT standard width [33.0–38.0], no tubercles around claw bases ..................................................... M. krzysztofi Kaczmarek & Michalczyk, 2007
Abbreviations: BT = buccal tube; SS = stylet support insertion point on the buccal tube.
Bartels P. J., Nelson D. R., Kaczmarek L. & Michalczyk L. 2014. The genus Milnesium (Tardigrada: Eutardigrada: Milnesiidae) in the Great Smoky Mountains National Park (North Carolina and Tennessee, USA), with the description of Milnesium bohleberi sp. nov. Zootaxa 3826 (2): 356 - 368. https: // doi. org / 10.11646 / zootaxa. 3826.2.5
Binda M. G. & Pilato G. 1990. Tardigradi di terra del fuoco e Magallanes. Milnesium brachyungue, nuova specie di tardigrado Milnesidae. Animalia 17: 105 - 110.
Johansson C., Miller W. R., Linder E. T., Adams B. J. & Boreliz-Alvarado E. 2013. Tardigrades of Alaska: distribution patters, diversity and species richness. Polar Research 32 (1): 18793. https: // doi. org / 10.3402 / polar. v 32 i 0.18793
Kaczmarek L., Michalczyk L. & Beasley C. W. 2004. Milnesium katarzynae sp. nov., a new species of eutardigrade (Milnesiidae) from China. Zootaxa 743 (1): 1 - 5. https: // doi. org / 10.11646 / zootaxa. 743.1.1
Kaczmarek L. & Michalczyk L. 2006. The Tardigrada fauna of Mongolia (Central Asia) with a Description of Isohypsibius altai sp. nov. (Eutardigrada: Hypsibiidae). Zoological Studies 45 (1): 11 - 23.
Kaczmarek L. & Michalczyk L. 2007. A new species of Tardigrada (Eutardigrada: Milnesiidae): Milnesium krzysztofi from Costa Rica (Central America). New Zealand Jounal of Zoology 34 (4): 297 - 302. https: // doi. org / 10.1080 / 03014220709510088
Land M., Musto A., Miller W. R., Starkey D. E. & Miller J. D. 2012. Tardigrades of the University of Central Arkansas Campus, Conway, AR. Southeastern Naturalist 11 (3): 469 - 476. https: // doi. org / 10.1656 / 058.011.0310
Londono R., Daza A., Caicedo M., Quiroga S. & Kaczmarek L. 2015. The genus Milnesium (Eutardigrada: Milnesiidae) in the Sierra Nevada de Santa Marta (Colombia), with the description of Milnesium kogui sp. nov. Zootaxa 3955 (4): 561 - 568. https: // doi. org / 10.11646 / zootaxa. 3955.4.7
Maucci W. 1991. Tre nuove specie di Eutardigradi della Groenlandia meridionale. Bollettino del Museo Civico di Storia Naturale di Verona 15: 279 - 289.
Maucci W. 1996. Tardigrada of the Arctic tundra with descriptions of two new species. Zoological Journal of the Linnean Society 116 (1 - 2): 185 - 204. https: // doi. org / 10.1111 / j. 1096 - 3642.1996. tb 02343. x
Meyer H. A. & Hinton J. G. 2010. Milnesium zsalakoae and Milnesium jacobi, two new species of Tardigrada (Eutardigrada: Milnesiidae) from the southwestern USA. Proceedings of the Biological Society of Washington 123 (2): 113 - 120. https: // doi. org / 10.2988 / 09 - 29.1
Meyer H. A. & Hinton J. G. 2012. Terrestrial Tardigrada of the Island of Barbados in the West Indies, with the description of Milnesium barbadosense sp. n. (Eutardigrada: Apochela: Milnesiidae). Caribbean Journal of Science 46 (2 - 3): 194 - 202. https: // doi. org / 10.18475 / cjos. v 46 i 2. a 8
Meyer H. A. 2015. Water bears (Phylum Tardigrada) of Oceania, with the description of a new species of Milnesium. New Zealand Journal of Zoology 42 (3): 173 - 186. https: // doi. org / 10.1080 / 03014223.2015.1062402
Michalczyk L., Welnicz W., Frohme M. & Kaczmarek L. 2012 a. Redescriptions of three Milnesium Doyere, 1840 taxa (Tardigrada: Eutardigrada: Milnesiidae), including the nominal species for the genus. Zootaxa 3154 (1): 1 - 20. https: // doi. org / 10.11646 / zootaxa. 3154.1.1
Michalczyk L., Welnicz W., Frohme M. & Kaczmarek L. 2012 b. Corrigenda of Zootaxa, 3154: 1 - 20 Redescriptions of three Milnesium Doyere, 1840 taxa (Tardigrada: Eutardigrada: Milnesiidae), including the nominal species for the genus. Zootaxa 3393 (1): 66 - 68. https: // doi. org / 10.11646 / zootaxa. 3393.1.6
Morek W., Blagden B., Kristensen R. & Michalczyk L. 2020 a. The analysis of inter- and intrapopulation variability of Milnesium eurystomum Maucci, 1991 reveals high genetic divergence and a novel type of ontogenetic variation in the order Apochela. Systematics and Biodiversity 18 (5): 1 - 19. https: // doi. org / 10.1080 / 14772000.2020.1771469
Moreno-Talamantes A., Roszkowska M., Garcia-Aranda M. A., Flores-Maldonado J. J. & Kaczmarek L. 2019. Current knowledge on Mexican tardigrades with a description of Milnesium cassandrae sp. nov. (Eutardigrada: Milnesiidae) and discussion on the taxonomic value of dorsal pseudoplates in the genus Milnesium Doyere, 1840. Zootaxa 4691 (5): 501 - 524. https: // doi. org / 10.11646 / zootaxa. 4691.5.5
Moreno-Talamantes A., Leon-Espinosa G., Garcia-Aranda M., Flores-Maldonado J. & Kaczmarek L. 2020. The genus Milnesium Doyere, 1840 in Mexico with description of a new species. Annales Zoologici 70 (4): 467 - 486. https: // doi. org / 10.3161 / 00034541 ANZ 2020.70.4.001
Pilato G. & Lisi O. 2016. Milnesium minutum and Milnesium sandrae, two new species of Milnesiidae (Tardigrada, Eutardigrada, Apochela). ZooKeys 580: 1 - 12. https: // doi. org / 10.3897 / zookeys. 580.6603
Pilato G., Sabella G. & Lisi O. 2016. Two new species of Milnesium (Tardigrada: Milnesiidae). Zootaxa 4132 (4): 575 - 587. https: // doi. org / 10.11646 / zootaxa. 4132.4.9
Schlabach S., Donaldson E., Hobelman K., Miller W. & Lowman M. 2018. Tardigrades of the Canopy; Milnesium burgessi nov. sp. (Eutardigrada: Apochela: Milnesiidae) a new species from Kansas, U. S. A. Transactions of the Kansas Academy of Science 121 (1 - 2): 39 - 48. https: // doi. org / 10.1660 / 062.121.0204
Surmacz B., Morek W. & Michalczyk L. 2019. What if multiple claw configurations are present in a sample? A case study with the description of Milnesium pseudotardigradum sp. nov. (Tardigrada) with unique developmental variability. Zoological Studies 58: 32. https: // doi. org / 10.6620 / ZS. 2019.58 - 32 Surmacz B., Morek W. & Michalczyk L. 2020. What to do when ontogenetic tracking is unavailable: a morphometric method to classify instars in Milnesium (Tardigrada). Zoological Journal of the Linnean Society 188 (3): 797 - 808. https: // doi. org / 10.1093 / zoolinnean / zlz 099
Tumanov D. V. 2006. Five new species of the genus Milnesium (Tardigrada, Eutardigrada, Milnesiidae). Zootaxa 1112 (1): 1 - 23. https: // doi. org / 10.11646 / zootaxa. 1122.1.1
Fig. 19. Milnesium quiranae sp. nov., paratype, ♀ (slide No. UNLPam 566-1). Buccal tube and claws of a young. A. Buccal tube and related structures. B. Legs I and II; the black arrow indicates the accessory points, the black arrowhead indicates the leg cuticular bar, the white arrowhead indicates a lunule. C. Leg III; the black and the white arrowheads indicate the same as in B. D. Legs IV with their claws. Scale bars = 10 µm.
Fig. 20. Milnesium quiranae sp. nov., holotype, ♀ (slide No. MCNS Tar.000024-3). A. Habitus. B. Smooth cuticle at the level of legs I. C. Smooth cuticle between legs III and IV. D. Smooth cuticle of the caudal end of the body. Scale bars: A = 50 µm; B–D = 10 µm.
Fig. 21. Buccal tube, peribuccal papillae and claws of senior specimens of Milnesium quiranae sp. nov. A, C–E. Holotype, ♀ (slide No. MCNS Tar.000024-3). B. Paratype, ♀ (slide No. UNLPam 560-1). A. Buccal tube and related structures. B. Reduced medioventral peribuccal papilla (black arrowhead). C. Leg II with its claws; the black arrow indicates the accessory points, the black arrowhead indicates the leg cuticular bar.D. Leg III with its claws. E. Legs IV with their claws, the white arrowhead indicates a lunule. Scale bars = 10 µm.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
1 (by felipe, 2022-06-07 12:33:16)
2 (by valdenar, 2022-06-07 14:44:59)
3 (by ExternalLinkService, 2022-06-07 14:55:20)
4 (by ExternalLinkService, 2022-06-07 15:01:56)
5 (by ExternalLinkService, 2022-06-07 16:20:43)
6 (by plazi, 2023-11-07 05:18:55)
7 (by ExternalLinkService, 2023-11-07 06:36:36)
8 (by carolina, 2023-11-24 13:08:09)
9 (by ExternalLinkService, 2024-11-27 15:17:28)
10 (by juliana, 2024-12-04 14:15:21)