Allodelphinidae
|
publication ID |
https://doi.org/ 10.7717/peerj.2321 |
|
DOI |
https://doi.org/10.5281/zenodo.5658612 |
|
persistent identifier |
https://treatment.plazi.org/id/E03BF937-AF24-FFF1-0484-FBECFA6FDB34 |
|
treatment provided by |
Plazi |
|
scientific name |
Allodelphinidae |
| status |
|
Systematics of Allodelphinidae
Our analysis recovered Allodelphinidae as a wellsupported subclade within a nodebased Platanistoidea, rooted in a polytomy with Squalodelphis fabianii and an unnamed subclade that includes Notocetus vanbenedeni C Phocageneus venustus C Platanistidae . Allodelphinidae in our study is supported by the following synapomorphies: rostral constriction anterior to the antorbital notch (character 9[1]); premaxillae in dorsal view contacting along midline for approximately half of the entire length of the rostrum and partially fused (character 14[3]); buccal teeth entocingulum absent (character 24[1]); greatest diameter of largest functional tooth <3% of greatest width of maxillae at postorbital processes (character 25[2]); angle of anterior edge of supraorbital process and the median line oriented anteromedially (character 35[1]); dorsolateral edge of internal opening of infraorbital foramen formed by maxilla (character 43[0]); posterolateral sulcus shallow or absent (character 57[1]); lack of premaxillary crest or posterior maxillary crest adjacent to nasals (character 72[0]); temporal fossa roofed over by lateral expansion of the maxillae (character 101[1]); palatines partially covered by pterygoid dividing it into medial and lateral exposures (character 121[1]); lateral lamina of palatine (character 122[1]); lateral end of groove for mandibular branch of trigeminal nerve wrapping laterally around posterior end of pterygoid sinus fossa and opening anteriorly (character 148[0]); lack of anterior bullar facet (character 172[1]); elevated caudal tympanic process of periotic with ventral and posterior edges forming a right angle in medial view (character 178[1]); tubular fundus of internal acoustic meatus (character 182[1]) angle between posterior process of periotic and long axis of pars cochlearis 135 from dorsal or ventral view (character 189[1]); and ventral surface of posterior process of periotic convex along a straight path perpendicular to its long axis (character 191[2]). Based on the published descriptions and illustrations provided by Kimura & Barnes (2016), the three allodelphinid taxa not included in our phylogenetic analysis (Allodelphis woodburnei, Ninjadelphis ujiharai, and Zarhinocetus donnamatsonae) each possess all of the allodelphinid synapomorphies presented by our analysis.
In their review of Allodelphinidae, Kimura & Barnes (2016) based their diagnosis of this group on comparative characters rather than phylogenetic synapomorphies. Many of these comparative characters can be readily observed in all platanistoids, such as the posteriorly extended lateral lamina of the pterygoid and palatine (except in species of Waipatia and Otekaikea where the palatine is poorly preserved or missing), and a tympanic bulla with elongated and pointed anterior process, among others. Nevertheless, our
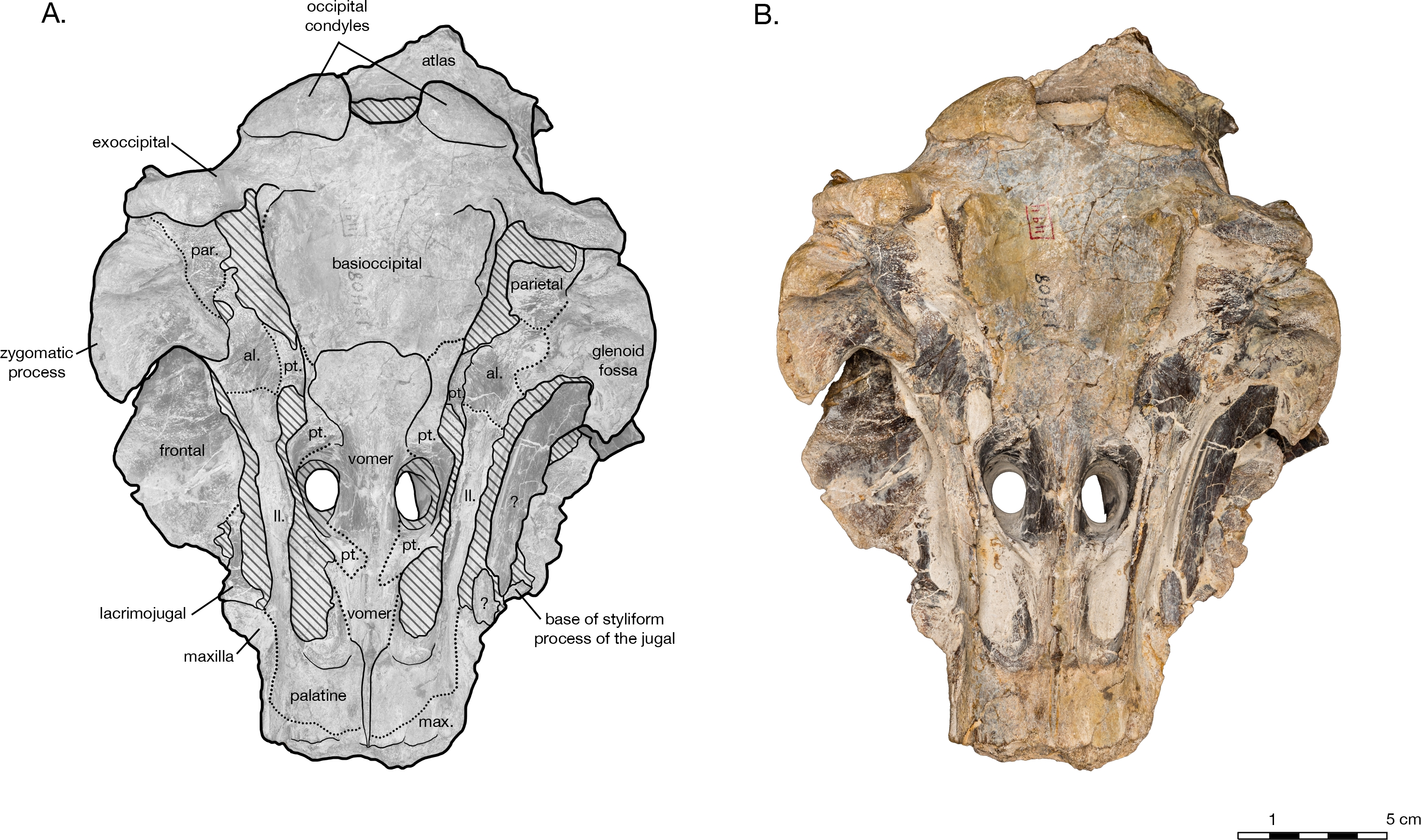
diagnosis is consistent with Kimura & Barnes (2016) ’s concept of Allodelphinidae with only two exceptions. First, Kimura & Barnes (2016) report that, in allodelphinids, the posterior ends of the premaxillae are separated from the lateral sides of the corresponding nasal bones, beginning with a more ‘‘primitive’’ state in Allodelphis pratti where only one premaxilla is separated from the corresponding nasal by a tiny exposure of maxilla, to further ‘‘derived’’ states in Ninjadelphis and Zarhinocetus where the premaxillae are further retracted anteriorly onto the facial region and away from the nasals. However, it is unclear in the more ‘‘primitive’’ state of Allodelphis whether the lack of contact between the premaxilla and nasal could be a result of diagenetic breakage, or individual variation. Furthermore, speculations on the more ‘‘derived states’’ in taxa such as Ninjadelphis, are based on specimens with incomplete premaxillae. In Goedertius oregonensis, the premaxillae are not separated from the nasals. This condition is likely also true for Arktocara yakataga: although the nasals are missing, the premaxillae directly abut the nasal fossa of the frontal, and therefore would most likely have been in direct contact with the nasals. Further extensive comparative work on allodelphinid taxa (including the multiple specimens housed at USNM that can readily be referred to Goedertius sp. (Fig. S2)) will help to clarify the distribution and diagnostic utility of these traits.
Second, Kimura & Barnes (2016) diagnosed Allodelphinidae by an absence of both the preorbital and postorbital lobe of the pterygoid sinus. Both fossae for the pre and postorbital lobe of the pterygoid sinus are unclear in the type specimen of Allodelphis pratti, in part due to obstruction by unprepared matrix. However, in Arktocara yakataga, though there is no obvious indication of a postorbital lobe of the pterygoid sinus, the deep and broad fossa surrounding the ventral infraorbital foramen and the sphenopalatine foramen anteromedial of the orbit suggests the presence of a preorbital lobe.
Originally assigned to Platanistidae by Wilson (1935), Allodelphis pratti was referred to the Platanistidae by Barnes (1977), and later Barnes (2006) erected a new group, Allodelphinidae , for it. However, in both instances, Barnes (1977) and Barnes (2006) did not provide an explanation for why the Allodelphinidae belong to the Platanistoidea. Of the 7 synapomorphies for Platanistoidea identified by our phylogenetic analysis, the Allodelphinidae possessed 4 of the 5 unequivocal characters: lateral groove or depression with profile of periotic becoming slightly to markedly sigmoidal in dorsal view (character 166[1]); anteroposterior ridge on dorsal side anterior process and body of periotic (character 167[1]); parabullary sulcus on the periotic weakly to strongly curved and cshaped (character 169[1,2]); and ventral surface of the posterior process of the periotic not flat along a straight path perpendicular to its long axis (character 191[1,2]). The fifth unequivocal character (147), could not be observed in any of the Allodelphinidae specimens. In addition, the type specimen of Ninjadelphis is the only allodelphinid specimen with an associated scapula, and it is missing the coracoid process. This agreement with Muizon’ s (1987) platanistoid synapomorphy the loss or reduction of the coracoid process of the scapula suggests that, though the process is still present in some putative platanistoids (i.e., Otekaikea huata), the character may still be relevant for diagnosing Platanistoidea ( Kimura & Barnes, 2016). We urge future studies on Allodelphinidae to not only include all available genera (if not putative species), but also to explicitly test phylogenetic hypotheses in a repeatable analytical framework.
Morphological comparisons
Of the 7 supporting synapomorphies for Platanistoidea in our study, none of the unequivocal synapomorphies are preserved and demonstrated on the skull of Arktocara. However, one equivocal synapomorphy is preserved in Arktocara: width of the premaxillae>50% of the width of the rostrum at the antorbital notch (character 51[1]). Though the type specimen of Arktocara lacks tympanoperiotics, it is closely allied with Allodelphis pratti, whose periotic shares three more of the platanistoid synapomorphies: presence of lateral groove or depression with the profile of the periotic becoming slightly to markedly sigmoidal in in dorsal view (character 166[1]); anteroposterior ridge developed on anterior process and body of periotic in dorsal view (character 167[1]); and a curved Cshaped parabullary sulcus (character 169[2]; see Fig. 9 View Figure 9 for illustration of the periotic synapomorphies on the type specimen of Allodelphis pratti). Therefore, in the absence of tympanoperiotics associated with new cranial material of Arktocara, we are confident that these elements would share many features with Allodelphis pratti, the sister taxon of Arktocara.
Overall, the allodelphinid that most resembles Arktocara in morphology is Allodelphis pratti ( Figs. 7 9 View Figure 7 View Figure 8 View Figure 9 ), originally described by Wilson (1935) from the Jewett Sand in Kern County, California, USA. Having examined the holotype consisting of a skull with associated rostrum and jaw fragments, other skull fragments, and a right periotic we note that the material included in the holotype actually belongs to more than one individual. For example, the holotype includes an isolated left postglenoid process, even though the holotype skull still has this feature intact. However, having examined the right periotic in relation to the periotic fossa, we are confident in the association of this element to the holotype skull. Any further study including the holotype of Allodelphis should take this caveat into consideration. Allodelphis is similar in size and shape to the type of Arktocara, with wide, hexagonally shaped craniums and postorbital widths within 2 cm of one another. In dorsal view, the two genera are alike in having their premaxillae rise above the maxillae for the entire length of the cranium from the level of the antorbital notch to the cranial vertex, forming an anteroposteriorly elongated and dorsally elevated plateau in relation to the broad, flat maxilla across the facial region. In both genera, this premaxillary plateau continues posteriorly to a tabular vertex, posterior to the external bony nares. The exposures of the frontals and nasals are symmetrical on the vertex, and there is no evident leftward skew or other facial asymmetry. The nasals are also transversely widened anteriorly, setting these two genera apart from all other allodelphinids. Kimura & Barnes (2016) showed photos of the holotype of Allodelphis pratti with a feature on the skull labeled ‘‘posterior dorsal infraorbital foramen.’’ However, having examined the holotype, we do not see any evidence of a posterior infraorbital foramina the featured labelled in the photo is a small break in the maxilla overlying the frontal. While it is possible that a posterior dorsal infraorbital foramen is hidden under the jaw fragment that is adhered to the right maxilla, we refrain from definitively stating any foramen exists. Both Arktocara yakataga and Allodelphis pratti have a nuchal crest weakly convex anteriorly, a widely open mesorostral canal anterior to the bony nares, the maxilla covering almost all of the frontal along the supraorbital process, and the posterior end of the basioccipital crest separated from the rest of the crest by a narrow crease.
The coded character state differences between Arktocara yakataga and Allodelphis pratti are listed in the Diagnosis section, above, although we provide more descriptive differences between these two taxa, as follows. First, Arktocara differs from Allodelphis in dorsal view by having: a deeper mesorostral canal anterior to the external nares; straight lateral margins of the premaxillae lateral and posterior of the external nares; no exposure of the maxillae on the vertex; a greater transverse constriction of the lateral margins of the maxilla/frontal anterior to the nuchal crest; a less extreme flaring of the posterior temporal crest along the occipital border; and more prominent dorsal infraorbital foramina, with posteriorly directed sulci. In lateral view, Arktocara shows a markedly reduced postglenoid process and zygomatic process of the squamosal, and a more posterolaterally directed postorbital process as opposed to a ventrally oriented process in Allodelphis. In ventral view, Arktocara has a more elevated vomerine keel. We argue that these differences, along with those coded in the phylogenetic analysis, provide the basis for Arktocara yakataga’ s status as a new genus of allodelphinid.
Arktocara also differs in clear ways from three allodelphinids (sensu Kimura & Barnes, 2016) that were not included in the phylogenetic analysis: Ninjadelphis ujiharai, Allodelphis woodburnei, and Zarhinocetus donnamatsonae. Arktocara differs from both Ninjadelphis ujiharai and Zarhinocetus donnamatsonae in having: a wider opening of the mesorostral canal, anterior to the external nares in dorsal view; anteroposteriorly straight lateral margins of the premaxillae both lateral and posterior of the external bony nares, in dorsal view; the posterior ends of the premaxillae extending posterior of the nasals; nasals expanding in width anteriorly rather than narrowing anteriorly; a reduced postglenoid process; and a broader extent of the maxilla above the supraorbital process of the frontal. Arktocara further differs from both Ninjadelphis ujiharai and Zarhinocetus donnamatsonae in lacking a dorsal depression on the base of the rostrum formed by ventromedially sloping of the premaxillae and maxillae, and lacking an asymmetrical skew to the vertex or nuchal crest.
Arktocara further differs from Ninjadelphis ujiharai in: lacking exposures of the maxillae on the vertex; lacking a glenoid fossa facing anteriorly as opposed to anteromedially; lacking widely diverging basioccipital crests; and lacking a depressed pit of the posterior end of the maxilla with an overhanging lip of the nuchal crest. Arktocara also differs from Zarhinocetus donnamatsonae in having: a more prominent and flaring temporal crest; a zygomatic process more tapered anteriorly in lateral view; the absence of a maxillary tuberosity on the lateral edge of the maxillary flange immediately anterior to the antorbital notch; no reduction of the maxilla on the supraorbital process to expose a thick band of frontal; and lacking a maxillary crest on the supraorbital process in dorsal view. Arktocara differs from Allodelphis woodburnei in having: a smaller and more anteriorly tapered zygomatic process; a reduced postglenoid process; the absence of a prominent fossa on each side of the sagittal crest on the supraoccipital; the premaxillae sloping medially towards the mesorostral canal on the posterior rostrum; and a glenoid fossa directed anteriorly rather than anteroventrally.
Geological & geographic significance
Today, Platanista gangetica is distributed in two subspecies across the Indus, Ganges BrahmaputraMegna and KarnaphuliSangu river systems of Southeast Asia, and remains highly threatened by human activities, including bycatch, fishing, and habitat modification (e.g., Braulik et al., 2014a). The fossil record of all other Platanistoidea demonstrates that the immediate relatives of Platanista gangetica comprise a morphologically diverse group of small to medium sized odontocetes that are distributed globally in marine sediments of Oligocene and Miocene age (see Bianucci et al. (2013) and Hulbert & Whitmore Jr (2006) for two exceptional occurrences of platanistid specimens in freshwater sediments of Peru and Alabama, respectively). There is no fossil record for the genus Platanista , but recent work on mitochondrial DNA haplotype diversity ( Braulik et al., 2014b) places the divergence between subspecies across at around 550,000 years ago (with 95% posterior probability 0.13 1.05 million years ago). The strong ecological disparity between Platanista’ s obligate freshwater lifestyle and the presumed marine lifestyle of all other named platanistoids ( Fig. 11 View Figure 11 ) implies some kind of differential evolutionary success for this group, with potentially higher extinction rates in Platanistoidea. Fordyce & Muizon (2001) first proposed that competition between platanistoids and early delphinioids may explain the strong difference in taxonomic richness observed in their fossil records, but this suggestion has never been tested in a rigorous framework ( Fordyce, 2003; Marx, Lambert & Uhen, 2016).
Platanistoids first appear in the fossil record in the late Oligocene, and reach peak richness in the early Miocene ( Kimura & Barnes, 2016; Tanaka & Fordyce, 2015a). The oldest platanistoids with solid age constraints are the waipatiids, all found in the Oligocene Miocene Otekaike Limestone of New Zealand ( Graham et al., 2000; Benham, 1935; Fordyce, 1994; Tanaka & Fordyce, 2014; Tanaka & Fordyce, 2015a). Based on both the lithology and the presence of agediagnostic planktic foraminifera and ostracod species, Waipatia hectori ( Benham, 1935) is the oldest reported waipatiid, from the uppermost Duntroonian Stage of the Otekaike Limestone, approximately 25.2 Ma ( Tanaka & Fordyce, 2015b). Arktocara is possibly very similar in age to Waipatia hectori, constrained to the Chattian Stage of the upper Oligocene in the Poul Creek Formation, approximately 24 29 Ma ( Perry, Garver & Ridgway, 2009). Unfortunately, the lack of robust locality data for either Waipatia hectori or Arktocara makes impossible to determine which is the oldest.
Arktocara is, however, very clearly the oldest known allodelphinid, expanding the previously reported age range of Allodelphinidae by as much as 9 million years ( Kimura & Barnes, 2016). Other allodelphinids span temporally from the early to middle Miocene, which largely matches the stratigraphic range of other platanistoid lineages ( Fig. 11 View Figure 11 ). Interestingly, Arktocara is among the oldest crown Odontoceti, reinforcing the longstanding view that the timing for the diversification for crown lineages must have occurred no later than the early Oligocene.
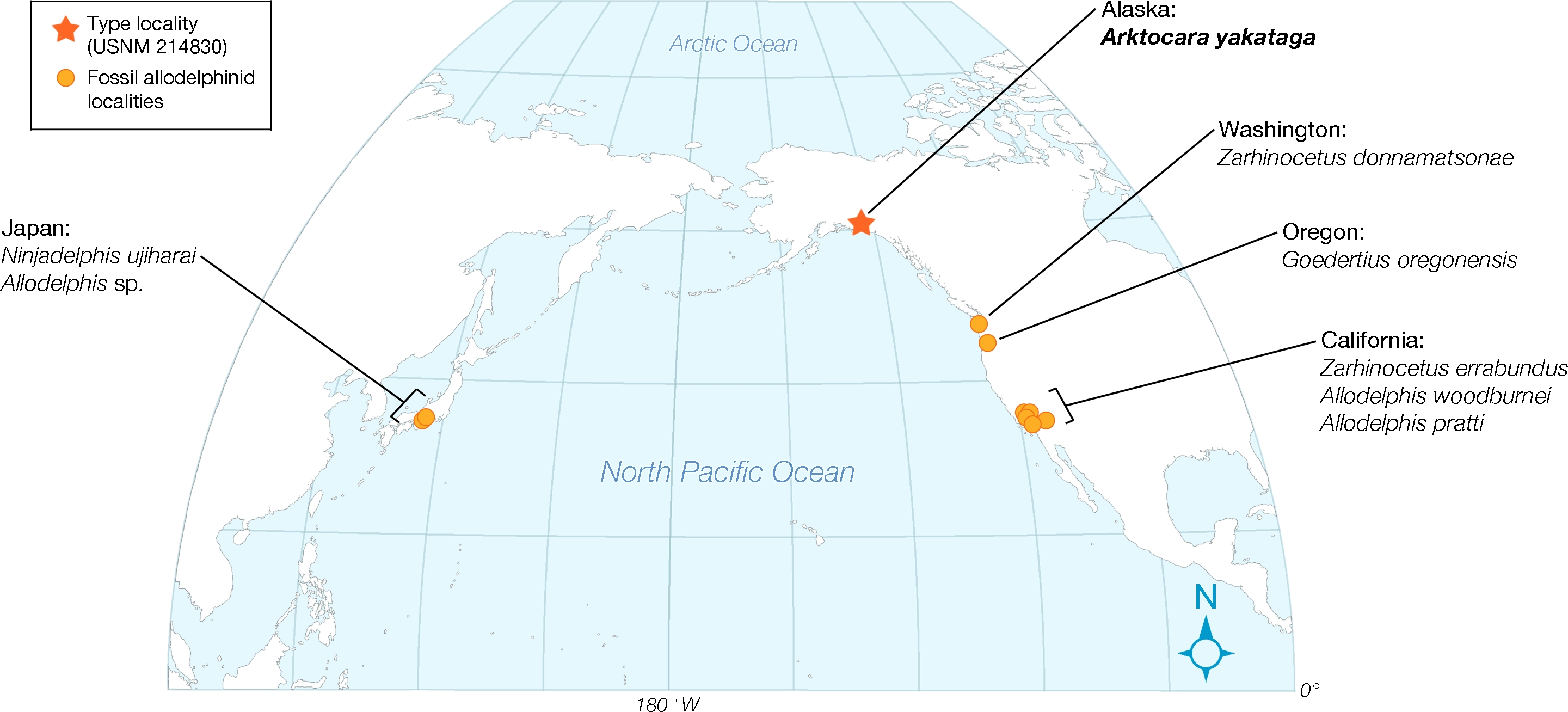
Lastly, Allodelphinidae appear uniquely limited, in terms of geography, to marine rocks of the North Pacific Ocean, with occurrences in Japan, Alaska, Washington State, Oregon, and California (see Fig. 12 View Figure 12 ; Kimura & Barnes, 2016). Arktocara expands this geographic range to subArctic latitudes. At approximately 60 N in the Yakutat City and Borough, Arktocara is the most northern platanistoid yet reported. The next most northern platanistoid reported is an incomplete and unnamed specimen from the late Chattian marine Vejle Fjord Formation in northern Denmark, approximately 56.7 N, 9.0 E ( Hoch, 2000).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Platanistoid |
|
Family |