Suffrica gus, Henrard, Arnaud & Jocqué, Rudy, 2015
|
publication ID |
https://doi.org/10.11646/zootaxa.3972.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:256EC29D-3CD6-499F-969A-C54B98DB041B |
|
DOI |
https://doi.org/10.5281/zenodo.6103038 |
|
persistent identifier |
https://treatment.plazi.org/id/DF1187D9-6D6E-FFF9-FF1F-72EDFB34FE26 |
|
treatment provided by |
Plazi |
|
scientific name |
Suffrica gus |
| status |
sp. nov. |
Suffrica gus View in CoL sp. nov.
Figs 64 – 96 View FIGURES 64 – 66 View FIGURES 67 – 69 View FIGURES 70 – 73 View FIGURES 74 – 81 View FIGURES 82 – 85 View FIGURES 86 – 89 View FIGURES 90 – 95 View FIGURE 96
Type material. Holotype. TANZANIA: ♂: Tanga, West Usambara Mts., Mazumbai Forest, 4°49’S 38°30’E, 1400 – 1800m, 11 – 20.XI.1995, sifting litter, C. Griswold, N. Scharff & D. Ubick ( ZMUC).
Paratypes. TANZANIA: 3♂ 6♀, same data as holotype; 1♂ 2♀ 6 j.: as previous ( ZMUC); 1♂ 1♀ 2 j: as previous, 11 – 19.XI.1995, pitfall traps ( ZMUC); 1♀: Tanga, East Usambara Mts., Amani, Mbomole Hill, 5°5.7’S 38°39’E, 1000m, 5 – 8.XI.1995, sifting litter, C. Griswold, N. Scharff & D. Ubick ( CAS); 1♂: as previous, Amani, forest, 5°5.7’S 38°38’E, 950m, 27.X – 9.XI.1995 ( ZMUC); 1♀: Tanga, East Usambara Mts., Sangarawe Forest, 5°6.5’S 35.7’E, 990m, 5 – 6.XI.1995, sifting litter, C. Griswold, N. Scharff & D. Ubick ( ZMUC); 2♂ 1♀: Tanga, West Usambara Mts., 4°49’S 38°30’E, 1400 – 1600m, 11 – 19.XI.1995, pitfalls, C. Griswold, N. Scharff & D. Ubick ( CAS); 8♂ 2♀ 2j.: Tanga, East Usambara Mts., Amani, 5°5.7’S 38°38’E, 950m, 28.X – 9.XI.1995, pitfalls, C. Griswold, N. Scharff & D. Ubick ( ZMUC); 19♂ 5♀ 5j.: Tanga, East Usambara Mts., Amani, 5°5.7’S 38°38’E, 950m, 28.X – 9.XI.1995, pitfalls, C. Griswold, N. Scharff & D. Ubick ( CAS; 2 ♂ 2♀ kept in MRAC); 2♂ 2♀ 2j.: as previous ( CAS); 1♀: Tanga, East Usambara Mts., Kwamkoro Forest Reserve, 5°10.9’S 38°35.8’E, 950m, 6.XI.1995, C. Griswold, N. Scharff & D. Ubick ( ZMUC).
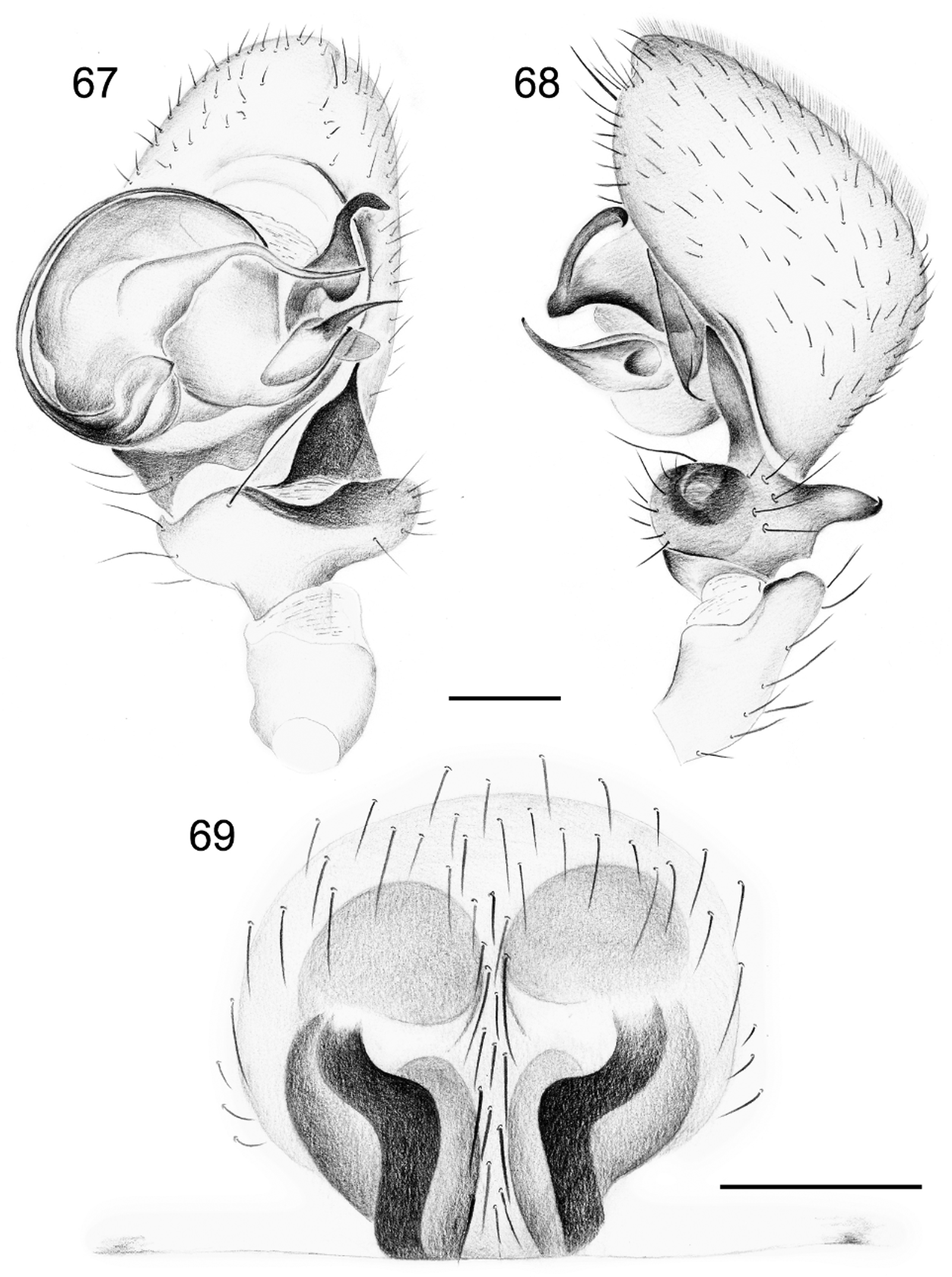
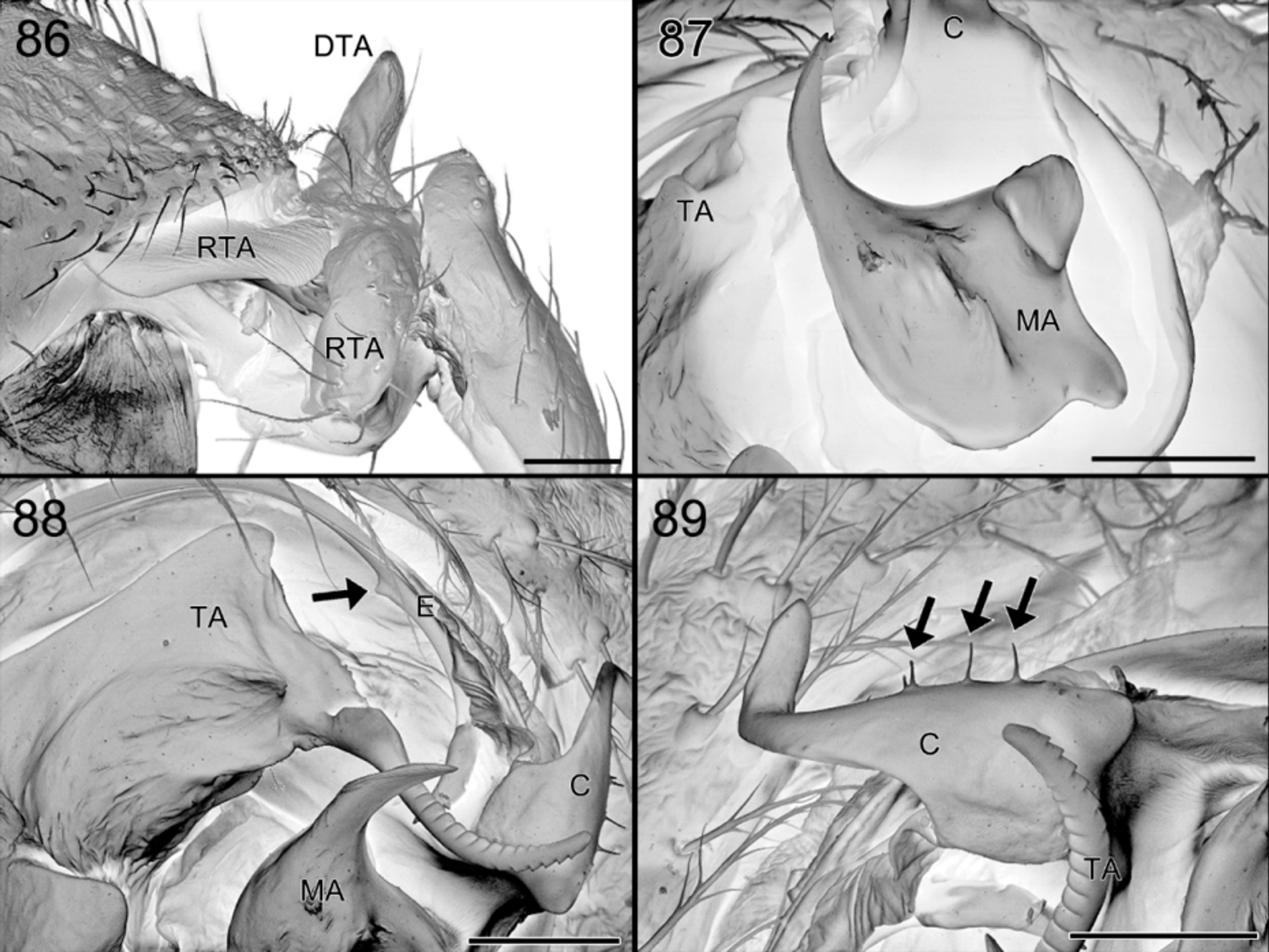
Diagnosis. Specimens of S. gus are recognized by the absence of a dark patch in front of the fovea ( Figs 64, 66 View FIGURES 64 – 66 ). The males are easily diagnosed by the row of thin spines on the conductor of the palp ( Fig. 89 View FIGURES 86 – 89 ), the thin and smooth distal prong of the MA ( Fig. 87 View FIGURES 86 – 89 ) and the abdominal modification: the central groove is shallow and delimited by whitish, narrowing and converging ridges and a small protruding pale brown scutum in front ( Figs 65 View FIGURES 64 – 66 , 74, 75 View FIGURES 74 – 81 ). The female differs from that of S. exotica by the less developed circular entrance in the frontal half of the epigyne and by the more angled copulation ducts ( Figs 69 View FIGURES 67 – 69 , 72, 73 View FIGURES 70 – 73 , 90 – 92, 94 View FIGURES 90 – 95 ).
Etymology. The species name is a noun in apposition derived from the first letter of the surnames of the collectors.
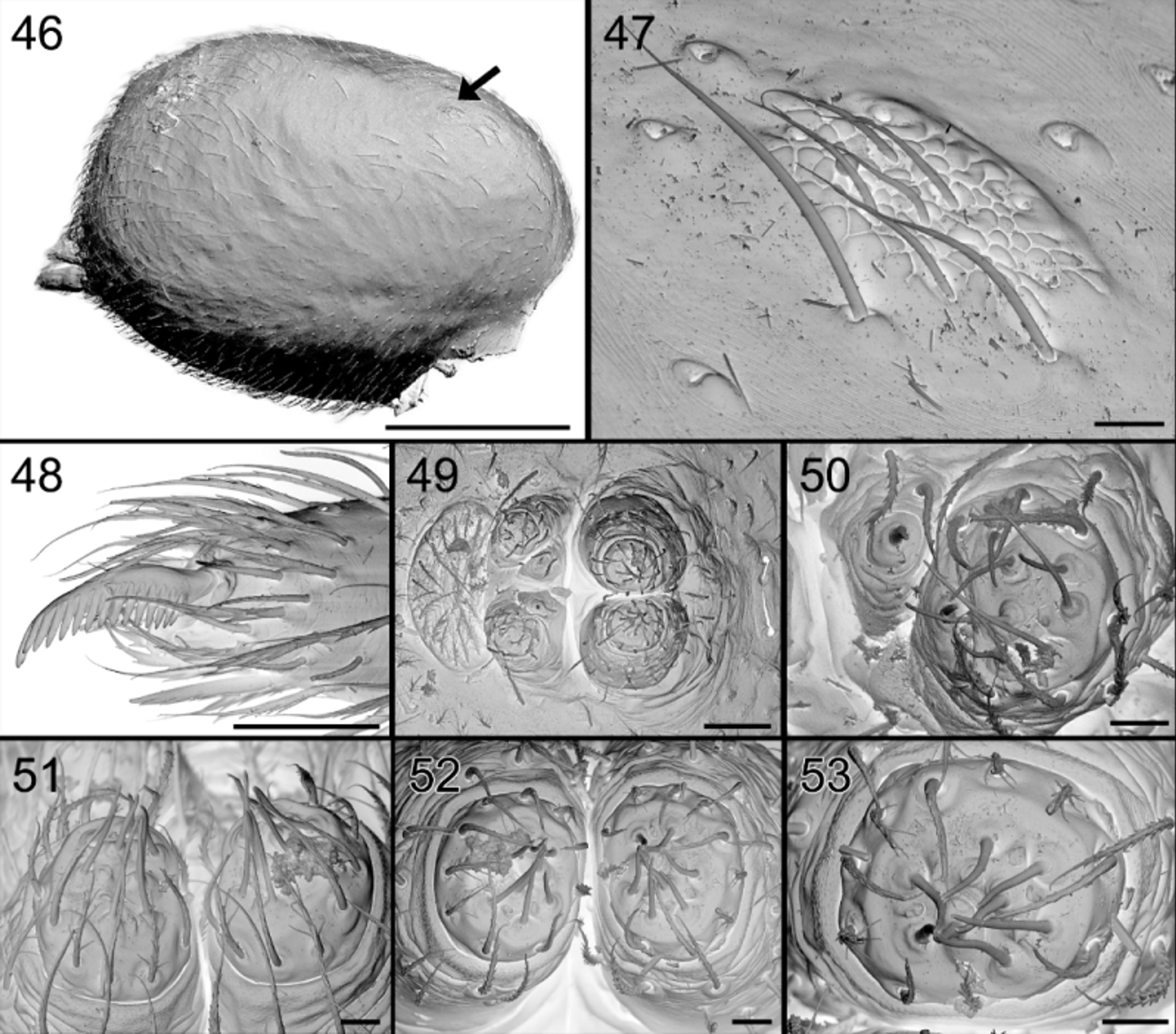
Description. Male ( Holotype). Total length 2.41, carapace 1.14 long, 0.78 wide and 0.50 high. Carapace ( Figs 64 View FIGURES 64 – 66 , 74 View FIGURES 74 – 81 ) very finely reticulated, medium brown, without dark area in front of fovea or if so, very faint; chelicerae and chilum pale brown; sternum yellow; legs yellow, femora slightly darker brownish. Clypeus 0.18 high. Eyes AME: 0.07: ALE: 0.08; PME: 0.08; PLE: 0.08; AME – AME: 0.04; AME – ALE: 0.05; PME – PME: 0.03; PME – PLE: 0.10. MOQ: width 0.16 in front, 0.18 at the back; 0.23 long.
Chilum single, 0.07 high, 0.12 wide; trapezoidal, slightly protruding in the centre, inferior margin concave. Sternum: 0.59 long, 0.53 wide.
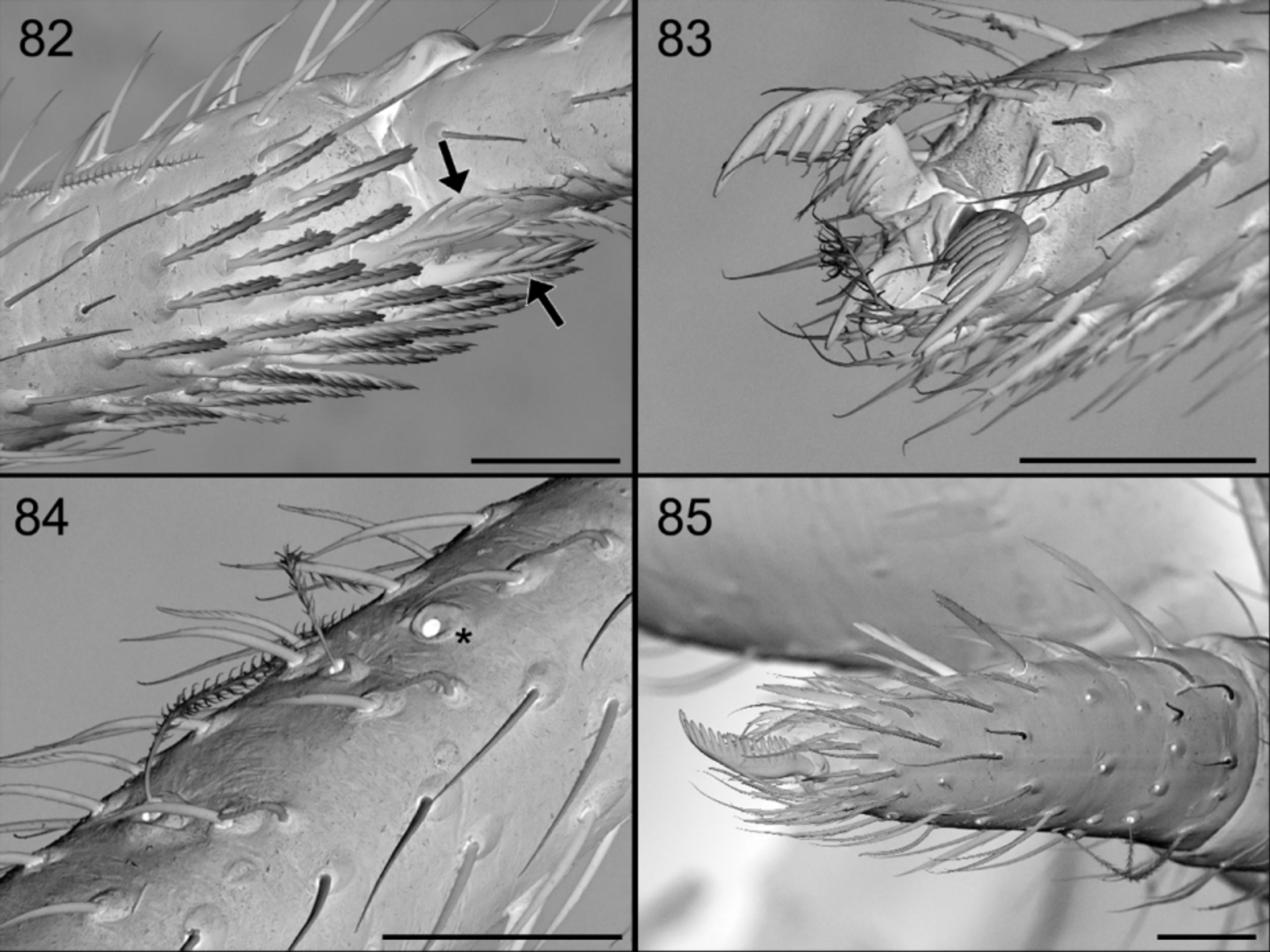
Legs: spination: Fe: I d 1 II d 1 III d 1 IV d2; T (all spines except dorsal at distal extremity): III d1 middle pl 1 v2 IV d1 at 2/3 pl 1 v 2; Mt (all spines at distal extremity): I v(pl)1 IIv(pl) 2 III dw 4 IV dw4. Metatarsi with three claws. Metatarsi II and III with preening brush of short chisel-shaped setae.
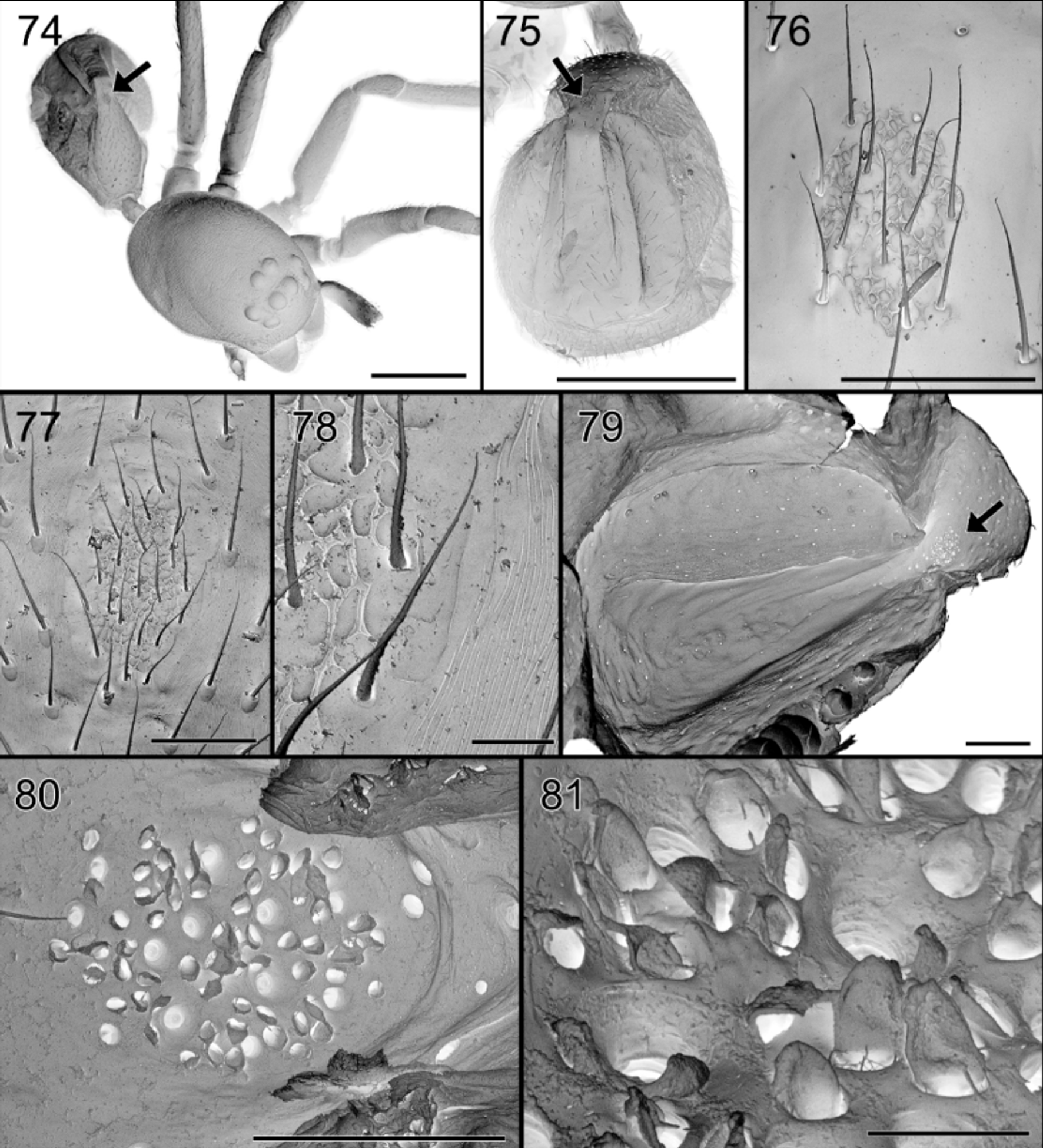
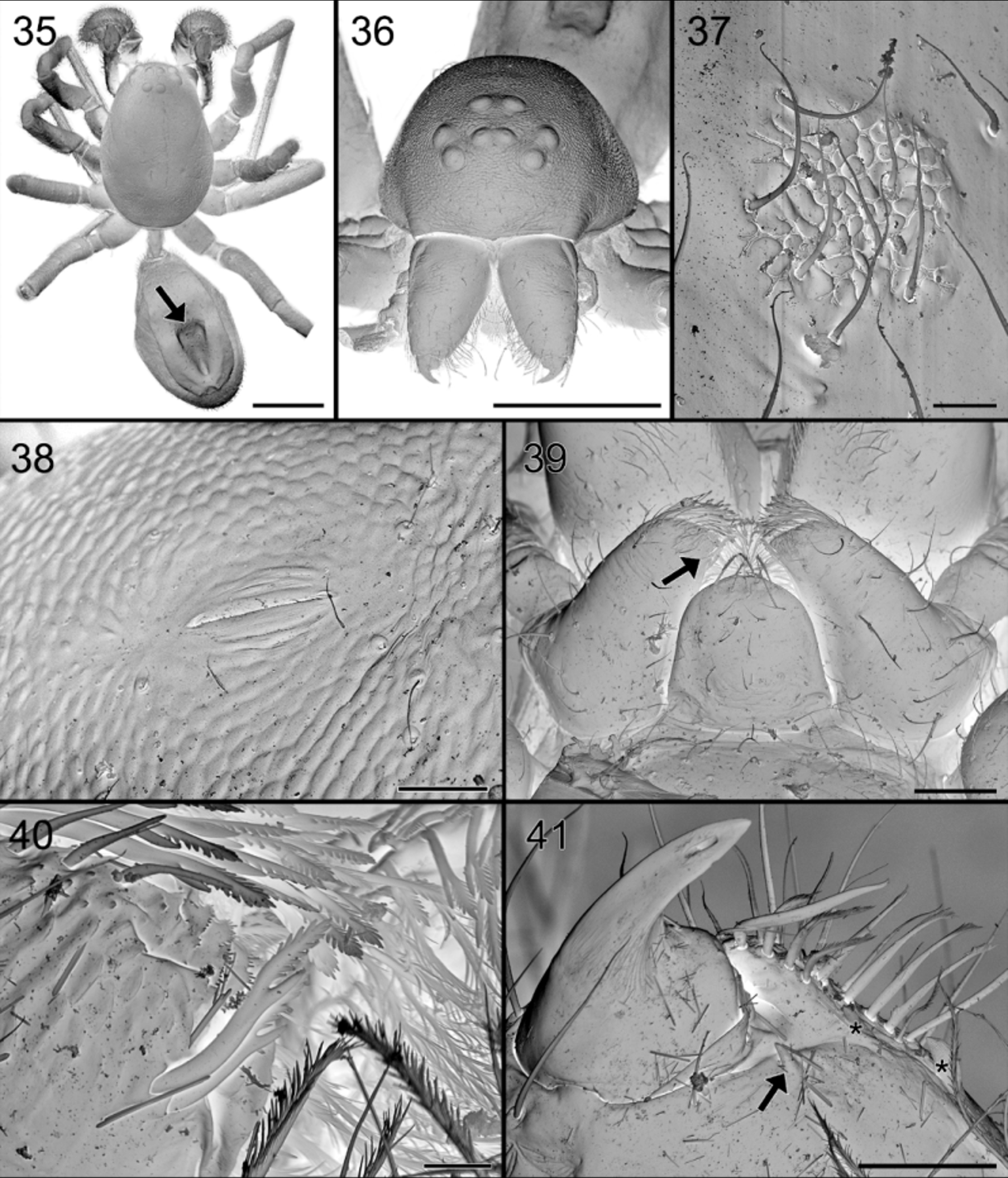
Leg measurements: Abdomen ( Figs 65 View FIGURES 64 – 66 , 74, 75 View FIGURES 74 – 81 ): dorsum with very faint brown sclerotization front of shallow central longitudinal dorsal furrow, delimited on sides by whitish, smooth, narrowing and converging ridges and small protruding scutum in front. Anteriorly provided with brownish orange oval, glandular outlet area with granulated tegument and thin needle-like seate ( Figs 65 View FIGURES 64 – 66 , 77 View FIGURES 74 – 81 ); internal view shows perforated oval area, with numerous pits, larger ones corresponding with the smooth setae, smaller ones accommodating the outlets of balloon-shaped glands ( Figs 79 – 81 View FIGURES 74 – 81 ). Sides sepia, venter pale, somewhat darkened around pedicel and in front of white spinnerets.
Palp. ( Figs 67, 68 View FIGURES 67 – 69 , 70 View FIGURES 70 – 73 , 77 View FIGURES 74 – 81 , 86 – 89 View FIGURES 86 – 89 ). Patella with fairly long dorsal extension; tibia with short dorsal and retrolateral anterior extensions; remainder of palp very similar to those of S. chawia and S. exotica except shape of distal part of conductor strongly curved and which is provided with a row of retrolateral needle-like spines; distal prong of MA slender.
Female ( Paratype CAS). Total length 2.84, carapace 1.35 long, 0.85 wide and 0.71 high. Carapace ( Fig. 66 View FIGURES 64 – 66 ), mouthparts and eyes as in male. Abdomen not modified; dorsum uniform dark sepia with numerous tiny white spots, with small pale scutum provided anteriorly with oval glandular outlet ( Figs 77, 78 View FIGURES 74 – 81 ); sides paler sepia, venter pale with two oblique dark patches extending from dark sides and dark spot in front of white spinnerets, surrounded by pale ring.
Chilum 0.07 high, 0.12 wide; trapezoidal, inferior margin straight.
Sternum: 0.64 long, 0.57 wide.
Legs: spination as in male. Leg measurements:
Palpal tarsus with 4–5 strong prolateral spines, with finely pectinated claw ( Fig. 85 View FIGURES 82 – 85 ).
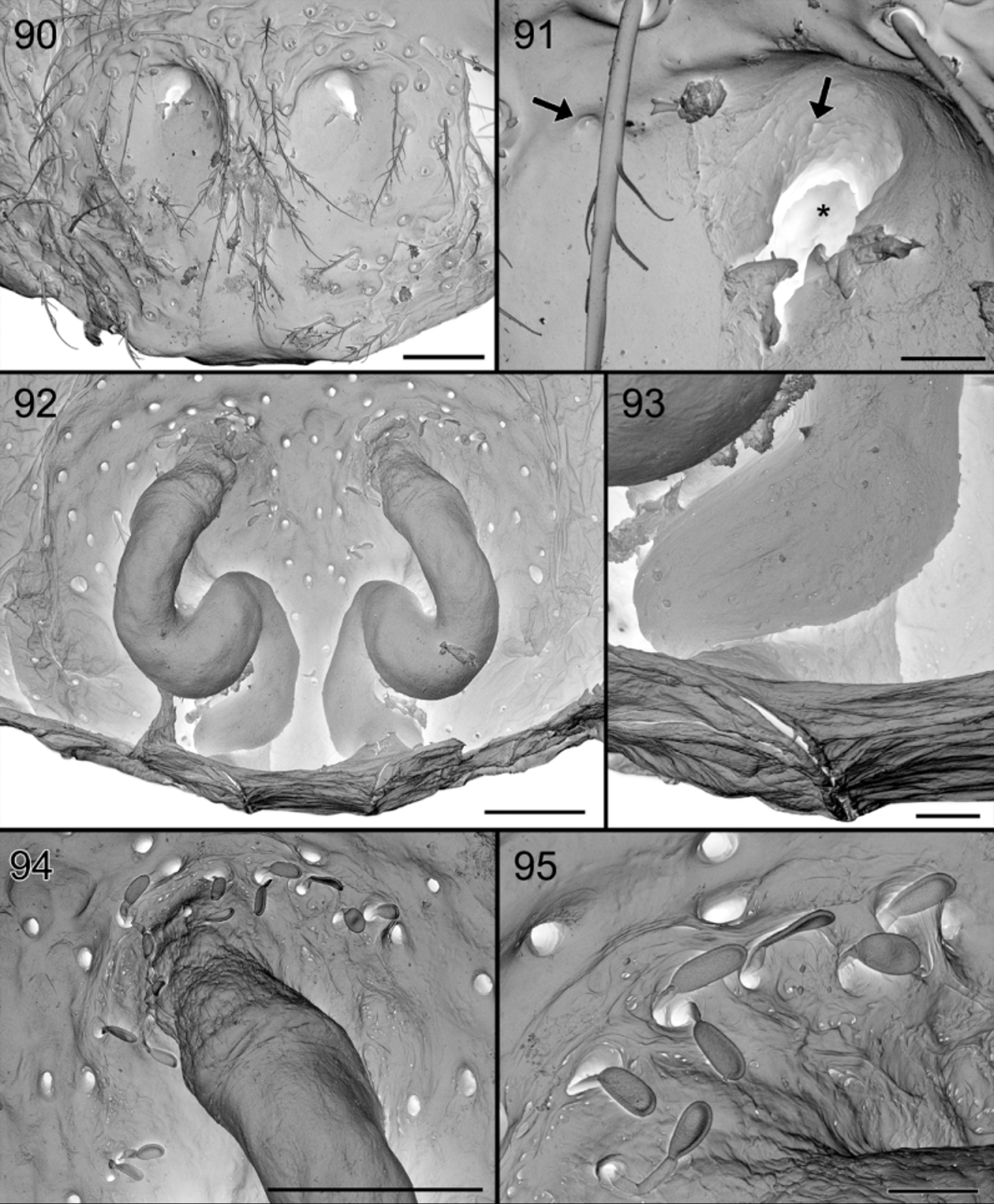
Epigyne. ( Figs 69 View FIGURES 67 – 69 , 72 View FIGURES 70 – 73 , 90, 91 View FIGURES 90 – 95 ). Provided with vase shaped medium brown pattern composed of entrance ducts and spermathecae seen in transparency. Vulva anteriorly with two membranous tube-shaped structures connected to the sinuous copulation ducts, leading to poorly defined elongated spermathecae ( Figs 73 View FIGURES 70 – 73 , 92 – 94 View FIGURES 90 – 95 ). Anterior membranous part internally with “lollipop” glands at its vicinity ( Fig. 95 View FIGURES 90 – 95 ) as in S. exotica but less numerous.
Variation. Total length varies between 1.9 and 2.5 for males (n= 24), between 2.3 and 2.7 for females (n= 19). The longitudinal dorsal ridges on the male abdomen may vary in width.
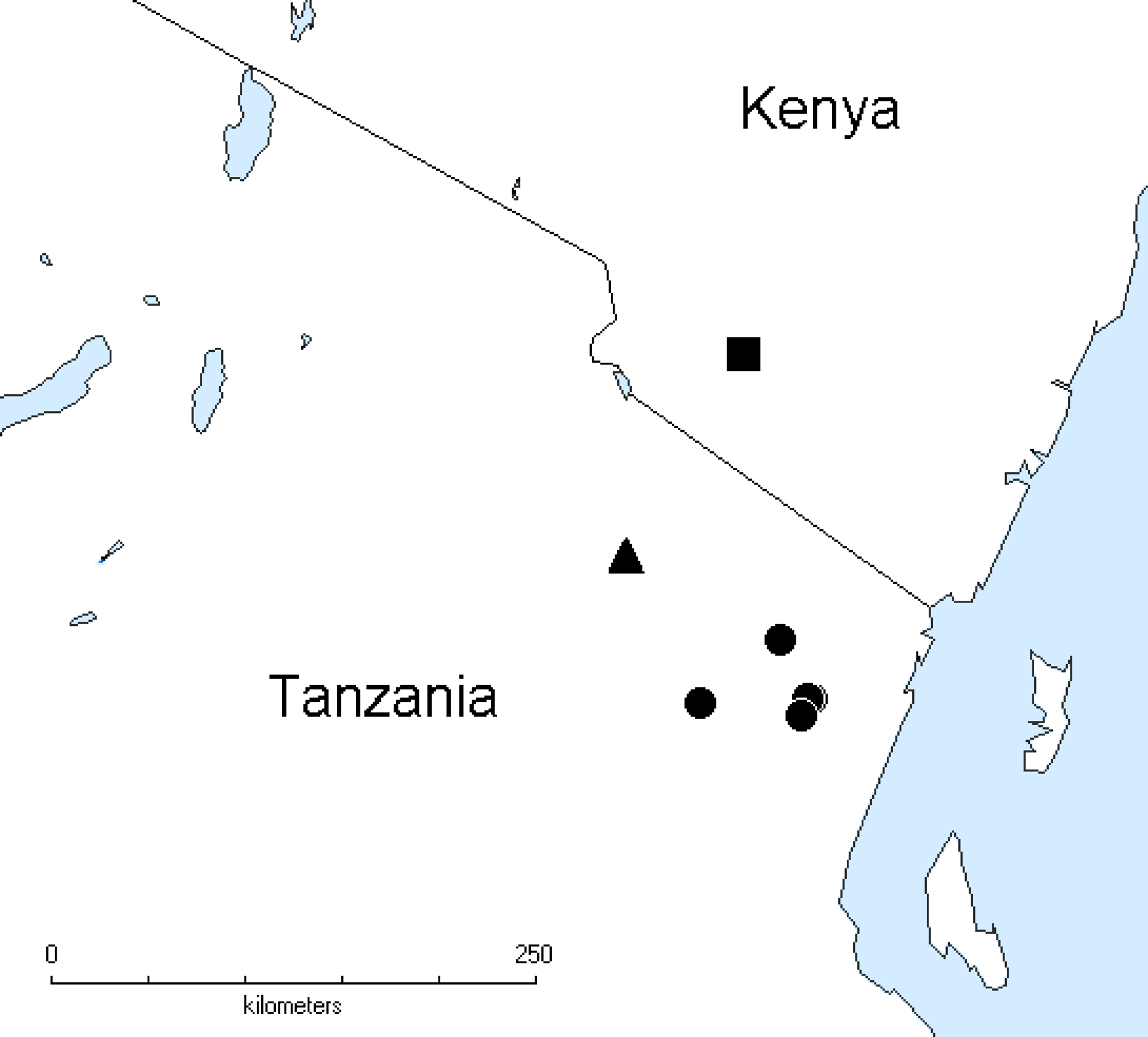
Distribution. Known from several localities in the Usambara Mts. in Tanzania ( Fig. 96 View FIGURE 96 ).
Discussion. The discovery of several species of a genus close to Suffasia may come as a surprise since the latter genus is widespread in Asia but has not yet been found in Africa. However, the localities where these species have been found, boast a particularly high biodiversity. The Eastern Arc Mountains are among the most diverse African regions ( Griswold 1991; Scharff 1993; Jocqué et al. 2013) and are famous for a number of very remarkable organisms often with unclear affinities, e.g. the damselfly Amanipodagrion gilliesi Pinhey, 1962 ( Dijkstra & Clausnitzer 2014) and the spiders Arctenus taitensis Polotow & Jocqué, 2014 ( Polotow & Jocqué 2014) and Ulugurella longimana Jocqué & Scharff, 1986 ( Jocqué & Scharff 1986) . The adjacent Mkomazi Game Reserve is the area with the highest number of species of Zodariidae in the world ( Haddad & Russell-Smith 2010; Russell- Smith & Jocqué, in press). Nevertheless, the distance between the core distribution of Suffasia and its relatives (e.g. Malayazodarion Ono & Hashim, 2008) in southwest Asia and that of Suffrica remains remarkable. There is, however, at least one other taxon with a similar distribution: a phasianid bird from Tanzania that has Indo-Malayan affinities (see Dinesen et al. 1994). These authors argue that opportunities for interchange of forest biota between tropical Africa and the Orient may well have existed in the mid-Miocene, before desertification and rifting of the Red Sea isolated the two regions. This implies that the Eastern Arc is an example of a mountain refugium as explained in Jocqué et al. (2013). The occurrence of S. chawia on only one of the Taita Hills, the Chawia Forest, is another indication that this is a relic distribution. The other forests in that chain of weathered old mountains were very thoroughly sampled (see Warui & Jocqué 2002; Polotow & Jocqué 2014) but do apparently not harbor this species.
Abdominal and epigynal glands. Spiders possess a wide variety of exocrine glands but most studies have focused on the silk and venom glands (e.g. Foelix 2011). However, whereas the role of these glands is quite obvious, the role of the secretions produced by other glands is not always clear. These secretions may be involved in sexual interactions including plugging of the epigyne, defensive behavior, predation and intraspecific communication (e.g. Eberhard 1980; Lopez 1998). On the ventral side of the abdomen, at the level of the epigastric furrow, epigastric glands have been found in both male and female spiders belonging to various families ( Lopez 1974, 1984; Kovoor et al. 1981). Their role is still uncertain and they appear to have a distinct function in each sex ( Pekár & Šobotník 2007).
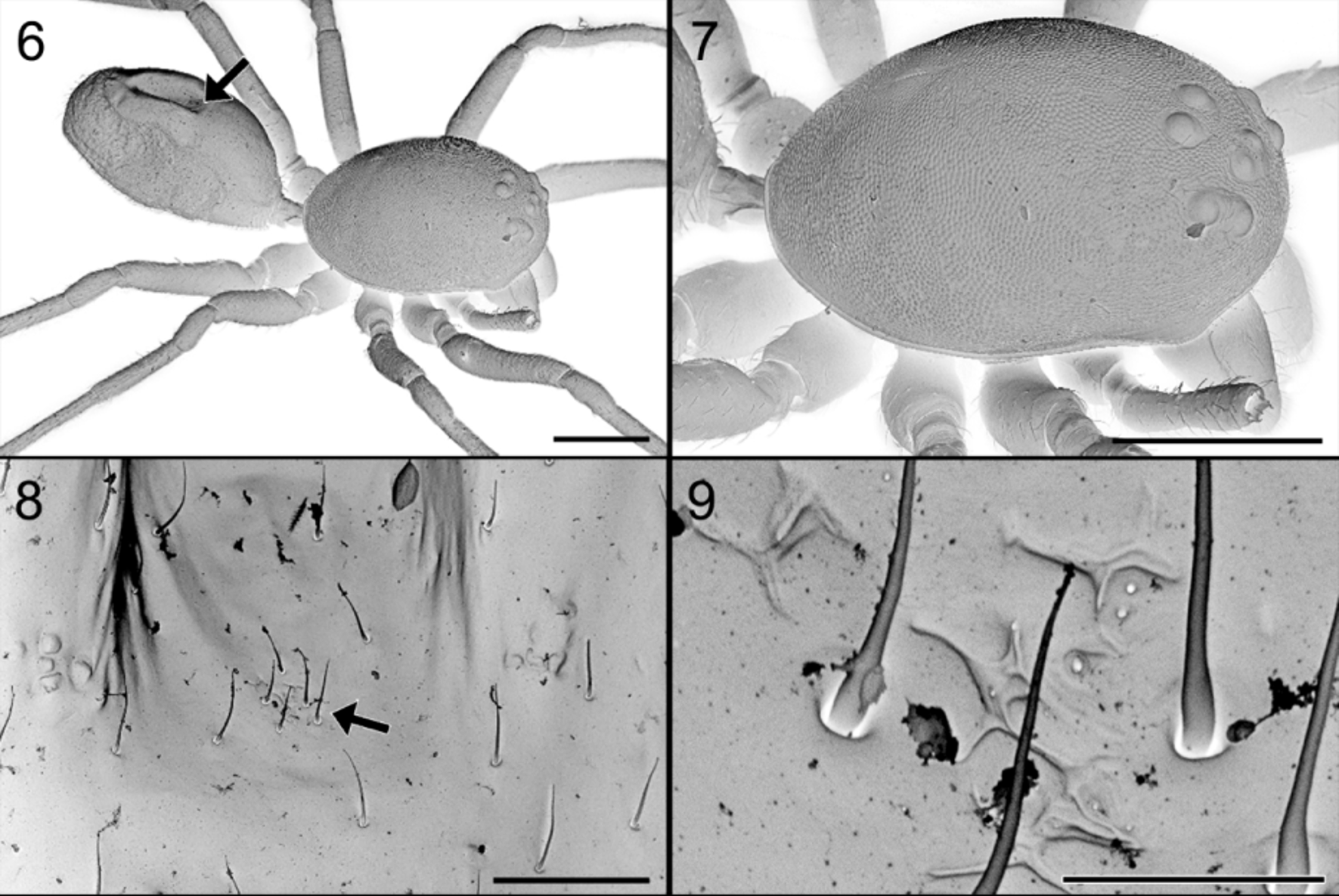
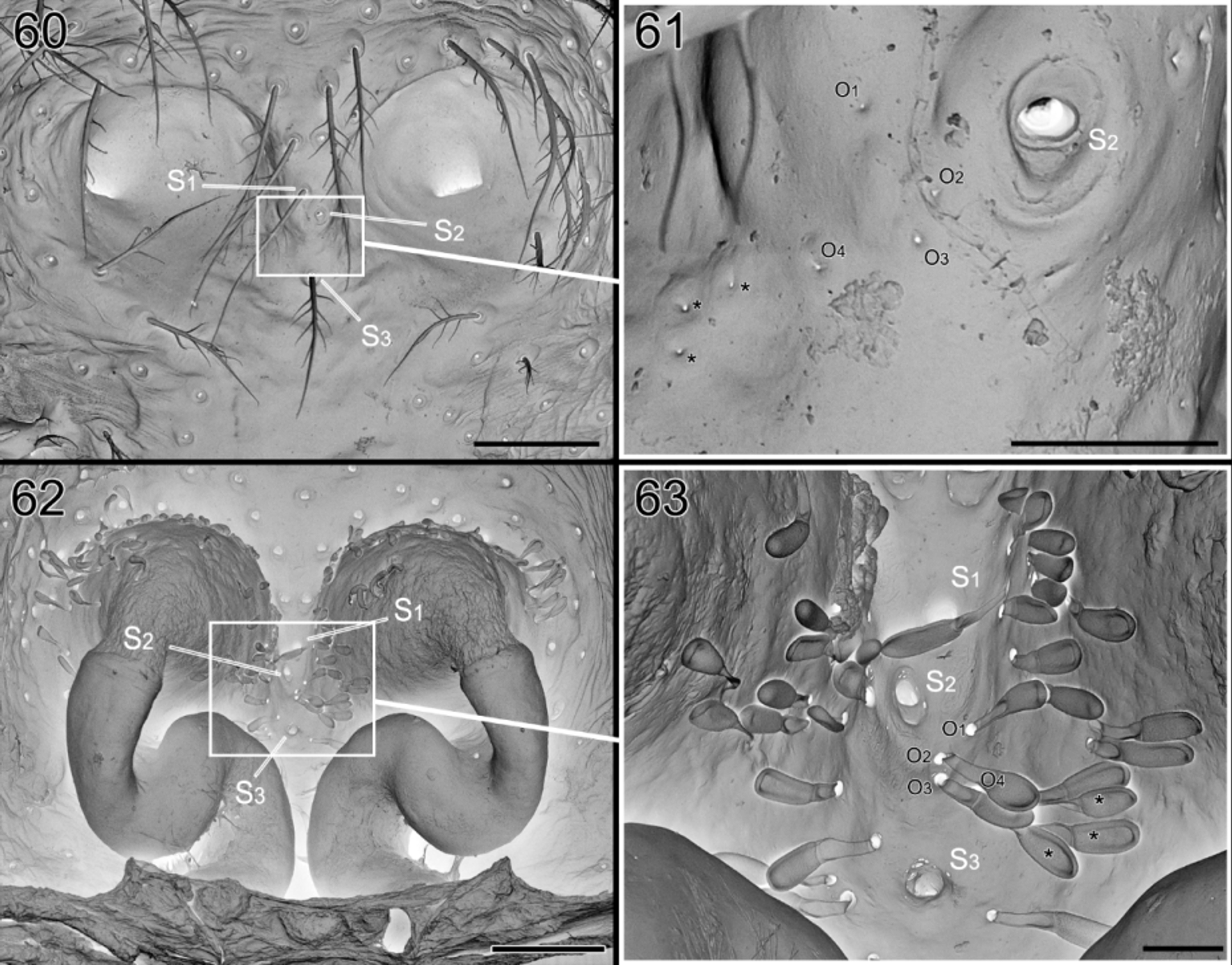
So far, no glands have been reported from the dorsum of the abdomen. In Suffrica , both male and female possess a glandular outlet on the dorsal side of the abdomen. This glandular outlet is visible as an oval area situated anterior to the mid-dorsum ( Figs 6 View FIGURES 6 – 9 , 35 View FIGURES 35 – 41 , 46 View FIGURES 46 – 53 , 65 View FIGURES 64 – 66 , 74, 75 View FIGURES 74 – 81 ) and has a granulate surface provided with small, smooth setae and a few tiny pores which are not always visible ( Figs 8, 9 View FIGURES 6 – 9 , 37 View FIGURES 35 – 41 , 47 View FIGURES 46 – 53 , 76 – 78 View FIGURES 74 – 81 ). Internally, the glandular outlet looks like a perforated area, with pits of different sizes: the larger pits correspond to the base of the setae and the smaller ones accommodate the outlet of a flat, oval gland ( Figs 79 – 81 View FIGURES 74 – 81 ). The flat appearance is doubtlessly the result of the digestion and drying processes during which the glands collapsed. They are probably either cylindrical or ampullate. A similar effect could also explain the flat appearance of the “lollipop” glands in the epigyne.
The epigynal glands appear to be organized in two parts: the proximal part is balloon-shaped, and the distal part tube-shaped or pedicel-like, entering in a large pit in the tegument and leading to a tiny pore in the outer surface. The size difference of the pores in the outer and inner side may be surprising. In Foelix (2010) (fig. 2.33C p.46), one can see a section of a dermal gland and the important constriction of the canal at the level of the outer cuticle. Furthermore, it could be traced for the “lollipop” epigynal gland (see Figs 60 – 63 View FIGURES 60 – 63 ).
The role of neither the dorsal abdominal glands nor the “lollipop” epigynal glands are known, and histological studies are needed to classify them.
Femoral Organ. The first observation of a femoral organ in Zodariidae was made by Tucker (1920) on several Diores species from South Africa. He mentioned a “distinct patch of short spiniform bristles” on the distal part of legs I – III, “giving the appearance of a sense organ”. The femoral organ was later found in different genera by Jocqué & Billen (1987) and Jocqué (1988). Their studies showed a remarkable range of variation in the structure of the gland outlet and of the bristles associated with it. A detailed study ( Pekár & Šobotník 2007) of the femoral gland itself in the genus Zodarion clearly demonstrated its role as an exocrine gland. Although it has been suggested that the gland produces a volatile compound, the composition of the secretion is unknown and the exact role of the femoral organs remains obscure. However, as most of the Zodariinae with these glands are specialised consumers of ants or termites, it is assumed that they may be involved in the predation of these insects ( Jocqué & Dippenaar-Schoeman 1992; Pekár 2004; Pekár et al. 2008). The gland might produce a secretion imitating the prey ant’s pheromone rendering the attack less hazardous ( Jocqué 1988) or a product that could prevent intraspecific attacks or cannibalism.
The femoral organ may be qualified as “simple” when it consists of a few smooth setae in a shallow depression provided with pores (i.e. Suffasia , Suffrica and Asceua (pers. obs.)) or may be considered “complicated” when provided with barbed setae (i.e. Zodarion , Diores , …). In rare cases it is encapsulated in a deep alveolus (e.g. Heradida ). Jocqué (1991) even postulated a hierarchical evolution of these femoral organs from the simple form towards the more complicated one. However, this hypothesis should be tested in a cladistic analysis. A detailed study of the morphology and distribution of the femoral organ in the subfamily Zodariinae is under way.
Until now it was thought that in most Zodariinae, only a single organ was present on each of the femora on which the gland is found (e.g. Pekár & Šobotník 2007). Here we clearly show that in Suffrica the gland is double and occurs on the pro- and retrolateral side of the distal part of the femora. A scan of other genera reveals that a similar set-up with a double gland is also present in Asceua , Suffasia and some undescribed species from Madagascar. It is worth to recall that in Jocqué (1991), Asceua was placed in the subfamily Storeninae because it has the chisel-shaped setae whereas Suffasia was part of the Zodariinae as it has the femoral organ. Actually, both these genera are characterized by the presence of preening brushes with chisel-shaped setae (characteristic for the Storeninae) and two femoral organs present on each femur. As a consequence, Jocqué (1992) fused the two subfamilies into the Zodariinae.
However, the external structure of the dual femoral organs appears different from the unique, barbed-setae ones in the other Zodariinae (sensu Jocqué 1991). Pekár & Šobotník (2007) also noted major differences in the internal structure of the gland between Suffasia (studied in Jocqué & Billen 1987) and Zodarion . This may provide an argument for the resurrection of the subfamily Storeninae, that will be tested in future analyses.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |