Amaeana Hartman, 1959
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3994.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:093B124E-58AE-4303-8C07-2D7B27E6AC38 |
|
DOI |
https://doi.org/10.5281/zenodo.6094890 |
|
persistent identifier |
https://treatment.plazi.org/id/DD7687BB-FFBD-FF9F-FF66-FAE0D844FA61 |
|
treatment provided by |
Plazi |
|
scientific name |
Amaeana Hartman, 1959 |
| status |
|
Genus Amaeana Hartman, 1959 View in CoL
Amaeana Hartman 1959: 495 View in CoL (replacement for Amaea Malmgren, 1866 View in CoL ; homonym).
Type species. Polycirrus trilobatus Sars, 1863 , designated by Hartman (1959).
Diagnosis. Transverse prostomium attached to dorsal surface of upper lip; basal part as thick crest, eyespots absent; distal part restricted to base of upper lip, with flaring lobes and frequently also mid-dorsal process. Buccal tentacles of three types, short tentacles thin, uniformly cylindrical, intermediate ones spatulate, long buccal tentacles spatulate or more specialised, with subdistal cylindrical swelling and pointed to blunt tip; apparently, tentacles with longitudinal groove only heavily ciliated at tips, with peduncle smooth or with poorly-developed longitudinal ciliary tract.
Peristomium forming lips; upper lip large, frequently circular and convoluted, folded into three lobes, short and swollen lower lip, only mid-ventral and usually button-like.
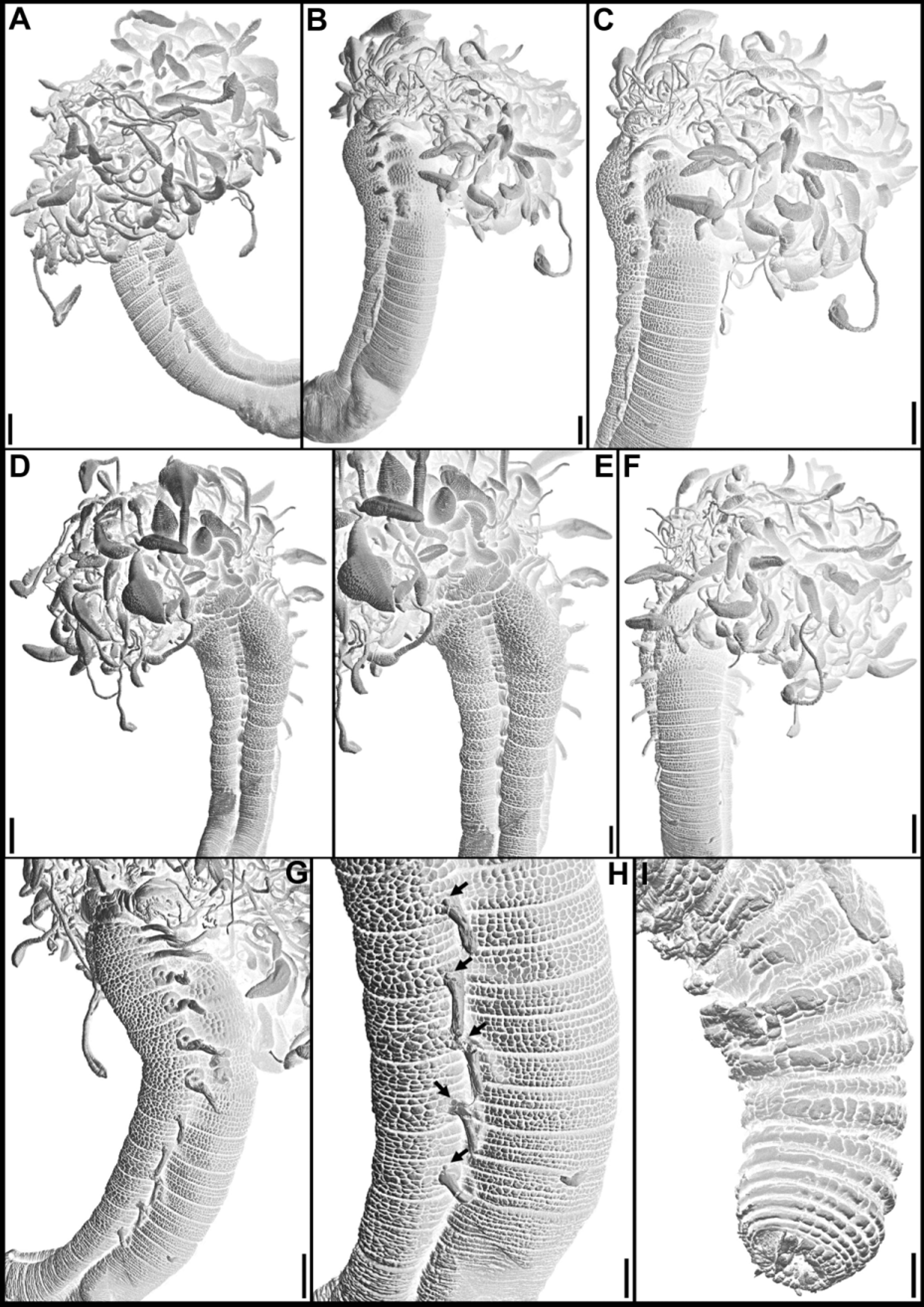
Segments biannulated throughout, segment 1 reduced, usually visible dorsally and ventrally, laterally covered by expanded prostomium (visibility of segment 1 strongly depends on state of preservation of specimens). Segment 2 distinctly narrower than following segments, separating “head” from remainder of body; segment 2 with rectangular or pentagonal mid-ventral shield at beginning of mid-ventral groove, sometimes extending anteriorly through segment 1 until near posterior margin of lower lip. Body wall papillate throughout, papillae distinctly larger and more abundant on ventro-lateral pads of anterior segments. Pads usually from segment 2 to last segment with notopodia.
Notopodia beginning from segment 3, extending for about 10 segments; notopodia elongate and bilobed, lobes about same size; notochaetae throughout usually with distinctly narrow wings, inconspicuous under higher magnifications of light microscopy, clearly visible under SEM; pinnate chaetae sometimes present.
Neuropodia beginning from midbody, after conspicuous gap of some achaetous segments between termination of notopodia and beginning of neuropodia; neurochaetae as distally tapering acicular spines, sometimes specialised at tips.
Nephridial and genital papillae present, usually anterior to bases of all notopodia. Pygidium smooth or with rounded ventral papilla.
Remarks. Amaeana was originally described as Amaea Malmgren, 1866 , but that name was preoccupied by a mollusk and was subsequently replaced with Amaeana ( Hartman 1959) .
The most important diagnostic character of Amaeana is the presence of neuropodia bearing tapering spines, always after a conspicuous gap of several achaetous segments between termination of notopodia and beginning of neuropodia.
In a recent phylogenetic study of Polycirridae, Fitzhugh et al. (2015) found species of Amaeana and Lysilla within an apomorphic clade within Polycirrus , and therefore none of these genera are monophyletic.
Nevertheless, the boundaries between these “genera” are well established morphologically: Polycirrus has notopodia bearing pinnate and/or winged notochaetae, usually broadly-winged sensu Fitzhugh et al. (2015), and neuropodia with uncini; Lysilla has notopodia only, bearing either pinnate or narrowly-winged, acicular notochaetae; and Amaeana has notopodia usually only bearing acicular notochaetae, although pinnate chaetae are present in two species, and neuropodia with spines, usually distally tapered.
Although these differences are conspicuous and easily recognizable in complete specimens, it is sometimes very difficult to distinguish between incomplete members of Lysilla and Amaeana if neuropodia are not present, and sometimes specimens from museum collections are misidentified, when members of both genera co-occur in the same locality.
Amaeana View in CoL currently includes 12 species, considering the seven new ones described herein. Two other species, A. colei ( McIntosh, 1926) and A. antipoda ( Augener, 1926) View in CoL are both treated as nomem nuda. Amaeana colei was described based on an anterior fragment of 5–6 segments ( McIntosh 1926); we could not locate that specimen, but, even if we did, it would still be undeterminable unless complete material from the type locality is found, as with only 5–6 segments it is not possible to determine if the specimen belongs to Amaeana View in CoL , Lysilla View in CoL , Polycirrus View in CoL or even Enoplobranchus View in CoL , since the differences between those genera are only visible from midbody. Therefore, A. colei is treated herein as nomen nudum.
In the case of A. antipoda View in CoL , material from Western Australia previously identified as belonging to this species is described herein as a new species, because the original description does not mention several characters important to distinguish this species from the remaining within the genus, and no material of this species from the type locality or nearby regions is currently available for study; a modern description for specimens of A. antipoda View in CoL from another region of New Zealand will be provided elsewhere (C. Glasby personal communication), but until that is available, it is not possible to compare A. antipoda View in CoL with several other species of this genus.
The distinction between the currently known species of this genus is based on ( Table 1):
(1) the presence or absence of a mid-dorsal process originating from the distal part of prostomium, which also occurs in some species of Lysilla View in CoL . Some species of Amaeana View in CoL have a clearly marked mid-dorsal process on the distal part of the prostomium (for instance, see Figs 9 View FIGURE 9 A, G; 14B, G), in others it is not as clearly marked, but it is still conspicuous ( Fig. 28 View FIGURE 28 B, E), while in still others such a prostomial process is apparently absent ( Figs 4 View FIGURE 4 B, E; 25F). However, although this character is easily visible in specimens missing buccal tentacles, it is frequently difficult to ascertain the presence of such processes in specimens with large numbers of buccal tentacles remaining ( Fig. 18 View FIGURE 18 B, D, G).
Hutchings & Glasby (1986) reported cocoon-like structures, possibly egg tubes, among the material the authors identified as A. trilobata View in CoL (in part herein described as A. ellobophora View in CoL sp. nov., see below); such structures are attached to the distal part of prostomium, in a position corresponding to the mid-dorsal prostomial process ( Fig. 1 View FIGURE 1 A–B). It seems likely that the presence of a mid-dorsal prostomial process may suggest they brood their embryos, although egg cocoons have not been observed in any other species of this genus to date.
Body Prostomial Modified Upper lip Lower ip Ventro- Number of Beginning of Number of Nephridial/ Pygidium
measurement (H process buccal lateral pads pairs of neuropodia achaetous Genital
= holotype; L = tentacles (segs); notopodia (seg.); number segments; papillae
lectotype; P = mid-ventral (segs); type (s) of spines and length (segs)
paratype) shield of of notochaetae morphology proportional
segment 2 to thorax
Amaeana View in CoL H: ~ 14.5 mm Rectangular to Apparently Circular Relatively 3–15; small, 12–13 (3–14 21; 8 slightly 5–6 (15–16 to 3 to 14–15 Unknown accraensis View in CoL long, 2.1 mm crescent-like absent, few large, rounded rectangular to (left side) or hooked, distally 20); shorter (all Augener, 1918) wide (incomplete) tentacles to oblong pentagonal 15 (right side); blunt notopodia)
remaining acicular and
on specimen pinnate
Amaeana View in CoL H: 20 mm long, 3 Present, Present Circular Rounded to 2–15; large, 10 (3–12); 21; 4–5 thin, 8 (13–20); 3-12 (all Smooth, angulus View in CoL sp. mm wide rectangular crescent-like pentagonal acicular distally tapered twice as long notopodia); ventral. spines, with enlarged on papilla
thinner knob- segs 6–10 absent
like tip
Amaeana View in CoL H: 12 mm long, Rounded to Present Circular Rounded to 3–12; small, 9–10 (3–11 or 22–23; 1–2 9–10 (12–13 3–11 or 12 With apheles View in CoL ~ 2.5 mm long squared rectangular, pentagonal 12); acicular thick, sharp to 21–22); (all rounded Hutchings, (incomplete). button-like (pinnate under spines twice as long notopodia); ventral) Longest specimen SEM) enlarged on papilla
examined ~15 segs 5–10
mm long, ~ 2 mm
wide
Amaeana View in CoL H: ~ 7 mm long, Rounded, Present Elliptical, Rounded, 2–13; small, 11 (3–13); 21; 2–3 spines, 7 (14–20); 3–13; With brasiliensis View in CoL sp. 1.5 mm wide raised higher than button-like pentagonal acicular distally blunt, or slightly enlarged on rounded. broad with flattened, shorter to segs 6–10 ventral
oblique tips same length papilla Amaeana View in CoL H: ~ 5 mm long, Absent Present Circular, Rounded, 2–11; small 9 (3–11); 15; 5–6 thin, 3 (12–14); 3–11 (all Unknown breviachaeta View in CoL 1.1 mm wide slightly button-like acicular tapering spines, shorter notopodia) nov. (incomplete) convoluted with hooked tip
Amaeana View in CoL H: 7 mm long, 1 Absent Tentacles Higher than Rounded, 2–12; large, 10 (3–12); 21; single spine 8 (13–20); 3–10, middle Crenulate, crassispinulata View in CoL mm wide missing on broad, button-like pentagonal acicular per slightly longer ones slightly ventral nov. type elliptical neuropodium, enlarged papilla
specimen basally broader, apparently
distally truncate absent
……continued on the next page Body Prostomial Modified Upper lip Lower ip Ventro- Number of Beginning of Number of Nephridial/ Pygidium measurement (H process buccal lateral pads pairs of neuropodia achaetous Genital = holotype; L = tentacles (segs); notopodia (seg.); number segments; papillae lectotype; P = mid-ventral (segs); type (s) of spines and length (segs) paratype) shield of of notochaetae morphology proportional segment 2 to thorax
Amaeana View in CoL H: 7 mm long, 2 Absent Apparently Elliptical, Rounded to 2–13; large, 11 (3–13); 26–27; 4-5 12–13 (14 to 3–10 (all Unknown dampierensis View in CoL mm wide absent, few higher than rectangular, pentagonal acicular sharp spines, 25–26); twice notopodia);. nov. (incomplete) tentacles broad button-like with flattened as long all small remaining tip
on specimen
Amaeana View in CoL H: 16 mm long, 3 Rounded, Apparently Elliptical, Relatively 2–12; large, 10 (3–12); 18; 8–11 thin, 5 (13– 17); 3–12 (all With ellobophora View in CoL sp. mm wide raised absent, few higher than large, rounded, pentagonal acicular distally tapered shorter, ½–2/3 notopodia) rounded. (incomplete). tentacles broad button-like spines, with as long ventral Longest specimen remaining thinner knob- papilla examined ~15 on specimen like tip
mm long, ~ 2 mm
wide (incomplete)
Amaeana View in CoL H: 17 mm long, 1 Absent Present Higher than Rounded to 3–12; large, 10 (3–12); 20; 2–3 thin, 7 (13–19); 3–12 or 13 With hsiehae View in CoL sp. nov. mm wide; longest broad, rectangular, pentagonal pinnate tapering spines, same length (all rounded paratype 34 mm almost button-like slightly or slightly notopodia in ventral long, ~ 3.5 mm rectangular expanded longer most specs), papilla wide subdistally, with enlarged on short oblique tip segs 6–10
Amaeana View in CoL H: ~ 65 mm long, Unknown, Present Circular Rounded, 2–14; small, 12 (3–14); 38; 8–9 to 12– 23 (15–37); 3–14 (all Crenulate, occidentalis View in CoL ~ 4 mm wide prostomium button-like pentagonal acicular 13 spines, much longer notopodia); with ventral Hartman, (incomplete) hidden by slightly enlarged on papilla 1944) buccal expanded segments 6–9 tentacles subdistally, with
oblique tip
Amaeana View in CoL L: ~ 31 mm long, Rounded to Absent Circular, Rectangular, 3–12; large, 10 (3–12); 16; 7–8 thin, 3 (13–15); 3–12 (all Unknown trilobata View in CoL (Sars, ~ 1.9 mm wide squared folded into short and broad pentagonal acicular tapering spines, shorter notopodia) 1863) (incomplete) three lobes with hooked tip
Amaeana View in CoL P: 7 mm long, 1 Rounded to Present Relatively Rounded to 3–14; small, 12 (3–14); 22–24; 1–2 7–9 (15 to 3–14 (all Unknown yirrarn View in CoL mm wide squared short, rectangular, rectangular to acicular tapered spines 21–23); notopodia) Hutchings, 1997 (incomplete) circular button-like pentagonal slightly
shorter
On the other hand, the specialised type of buccal tentacles apparently originates exclusively in mid-dorsal position, from the mid-dorsal process when this is present, or an equivalent position in species in which such a prostomial process is absent ( Fig. 4 View FIGURE 4 B, D–F). However, other types of tentacles also originate from the mid-dorsal process, not only the specialised type (see Fig. 9 View FIGURE 9 A, G).
(2) the morphology of the long buccal tentacles varies: they may be with highly specialised tips, or only distally expanded, spatulate, without further modifications, similar to those of other polycirrids. However, buccal tentacles are frequently missing in type material and the specialised tentacles are usually much fewer in number than remaining ones. For that reason, the absence of this type of tentacle in some specimens does not necessarily indicate that they were not originally present; however, in the cases in which many buccal tentacles are present with no specialised ones ( Fig. 2 View FIGURE 2 E), we have assumed this type of buccal tentacles is absent.
The specialised buccal tentacles of some species of Amaeana View in CoL are quite remarkable. Although we have only examined the buccal tentacles of A. occidentalis View in CoL and A. hsiehae View in CoL sp. nov. under the SEM, the tentacles of the other species in this genus look similar to those, at least under stereomicroscope. In A. occidentalis View in CoL all types of tentacles are thin, with an uniformly wide peduncle and specialised tip, from elliptical or spherical, to spatulate or more elaborate, with large subdistal expansion and tapered tip; a ciliated groove is only present distally, the peduncle being smooth or nearly so ( Figs 20 View FIGURE 20 A–G; 21A–G); the specimen of A. hsiehae View in CoL sp. nov. examined has few tentacles remaining, but exhibiting a similar pattern. This means that these worms most likely insert the tips of the tentacles inside the mouth, or brush them against the highly ciliated upper lip to remove food particles gathered from the substrate, rather than conveying those particles along the entire tentacle.
(3) the number of pairs of nephridial/genital papillae and notopodia: nephridial/genital papillae occur at the anterior bases of all notopodia, from segment 3 to last one, nephridial papillae on segments 3–5, genital papillae from segment 6 to last thoracic chaetiger ( Nogueira et al. 2010, 2013). There is a strong correlation between the number of pairs of papillae and the pairs of notopodia, so the variation found in the number of pairs of notopodia present is also observed in regards to the number of pairs of nephridial/genital papillae. However, sometimes papillae of anterior- and/or posterior-most thoracic chaetigers are distinctly shorter than remaining ones ( Figs 20 View FIGURE 20 G–H; 22G) and they may be difficult to distinguish from the numerous glandular papillae which cover the ventral surface of ventro-lateral pads. On the other hand, in some species, genital papillae of some mid-thoracic segments are distinctly larger than those of remaining segments ( Figs 5 View FIGURE 5 P–Q; 19A, C; 20G–H).
(4) the number of segments with ventro-lateral glandular pads: this character is also strongly correlated with the number of pairs of notopodia, as the paired glandular ventro-lateral pads usually extend from segment 2 to the last with notopodia, frequently with fewer papillae, restricted to the posterior annulation of each segment, on last chaetigers with notopodia. The only species of Amaeana View in CoL with ventral pads extending beyond the termination of the notopodia is A. angulus View in CoL sp. nov., which has notopodia until segment 12 and pads extending to segment 15, although the last three pairs are distinctly smaller ( Fig. 14 View FIGURE 14 A, E).
(5) the types of notochaetae present: most species of Amaeana View in CoL have narrowly-winged notochaetae sensu Fitzhugh et al. (2015) in both rows; those chaetae are acicular, with inconspicuous wings under higher magnifications of light microscopy, but visible as fine hairs under SEM. Among members of two species, however, pinnate chaetae are present, in the posterior row of notochaetae in A. accraensis View in CoL ( Fig. 27 View FIGURE 27 A–C), and in both rows in A. hsiehae View in CoL sp. nov. ( Fig. 6 View FIGURE 6 A–B; 8B–F). Some species, such as A. apheles View in CoL , have notochaetae that strongly resemble pinnate chaetae under SEM, but this is not visible under higher magnifications of light microscopy (compare Figs 9 View FIGURE 9 I–J, 11A–E, to see those chaetae under light microscopy and SEM, respectively), and so these chaetae are also treated here as acicular notochaetae, because it is not practical to diagnose a species by characters only visible under the SEM.
(6) the extension of the achaetous gap between termination of notopodia and beginning of neuropodia: this region is often swollen, fragile, with segments distinctly longer than those with notopodia and with thinner body wall. Segmentation is poorly marked in this area, frequently rendering it difficult to count the number of segments.
However, the whole concept of segmentation is challenged here, because terebelliforms have no internal septa other than the gular membrane, between segments 4 and 5, and if segmentation is also not clear externally, then the counting of “segments” between termination of notopodia and beginning of neuropodia may be a misconception. It seems more reasonable to measure this region in relation to the length of the region with notopodia, and frequently it is at least as long as that part of the body, but this proportion is also strongly dependent on the state of preservation of specimens, together with muscular contraction at the time of fixation. Both measurements are provided herein for each species, the number of achaetous “segments” and the length relative to the region with notopodia.
Posteriorly, this region usually tapers abruptly to an uniformly cylindrical abdomen, and neuropodia first occur somewhere among these tapering segments and the first ones of the uniformly cylindrical region, depending on the species.
(7) the number of spines per neuropodium and their morphology: considering we have worked with type material for the present study, we could not prepare slides of several neuropodia of our specimens, to determine the variation in the number of neuropodial spines per neuropodium. Instead, except for some of the species herein described, we mounted two slides per specimen, one from an anterior neuropodium, a few segments after the beginning of neuropodia, and another from a posterior segment; in other cases we mounted a single slide, usually from a mid-abdominal segment. However, only A. occidentalis View in CoL and A. ellobophora View in CoL sp. nov. exhibit variation greater than 1–2 spines per neuropodium between anterior and posterior abdomen. There was little to no variation between members of the same species in cases where we were able to study more than one specimen of each taxon. Therefore, we believe a great variation on the number of spines per neuropodium, as reported sometimes in the literature (see below), is possibly due to the presence of several species being confused.
When more than 3 spines are present per neuropodium, these usually have uneven lengths and only 1–2 spines protrude from each neuropodium (for instance, compare Figs 15 View FIGURE 15 M–O and 17C–D, showing neuropodia of A. ellobophora View in CoL sp. nov. under light microscopy and SEM, respectively). With the exception of A. crassispinulata View in CoL sp. nov., the neuropodial spines are straight and distally tapered, but frequently the spines are further modified to a narrower knob-like or slightly hooked tip, sometimes flattened and concave, spoon-shaped ( Figs 3 View FIGURE 3 B, D–E, H; 6C– E; 8I –L; 11H–I; 19G–H; 24C, E, H–I; 27D). This is difficult to clearly visualize because it depends critically on the alignment of the spines on slides; further, in compound microscopes the highest magnification does not allow for a proper analysis of this character, while under the SEM the tips of the spines are often not visible, as these structures do not always protrude from the neuropodial pinnules, and when they do, frequently only a short part of the tip is exposed ( Figs 11 View FIGURE 11 F–I; 17C–D; 22A–F; 29 H–J).
Other characters also useful to distinguish between the species of Amaeana View in CoL are the shapes of upper and lower lips, and of the mid-ventral shield of segment 2. The upper lip is usually circular, but it may be elliptical, higher than wide, as in A. brasiliensis View in CoL sp. nov. ( Figs 28 View FIGURE 28 A, C–D, F; 29B–C, E–F) or almost rectangular, as in A. hsiehae View in CoL sp. nov. ( Figs 5 View FIGURE 5 A, E–F, I–K, M; 7C, G, I). The lower lip and the mid-ventral shield of segment 2 may be small or large, swollen, but this is also strongly dependent on the state of preservation of the specimens, with some intraspecific variation observed.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Terebelliformia |
|
Family |
Amaeana Hartman, 1959
| Nogueira, João Miguel De Matos, Carrerette, Orlemir & Hutchings, Pat 2015 |
Amaeana
| Hartman 1959: 495 |