Tidarren sisyphoides ( Walckenaer, 1842 )
|
publication ID |
https://doi.org/ 10.1080/00222930600940993 |
|
persistent identifier |
https://treatment.plazi.org/id/DC30557E-B336-A719-FE15-FD89FDE7FD9F |
|
treatment provided by |
Felipe |
|
scientific name |
Tidarren sisyphoides ( Walckenaer, 1842 ) |
| status |
|
Tidarren sisyphoides ( Walckenaer, 1842) View in CoL
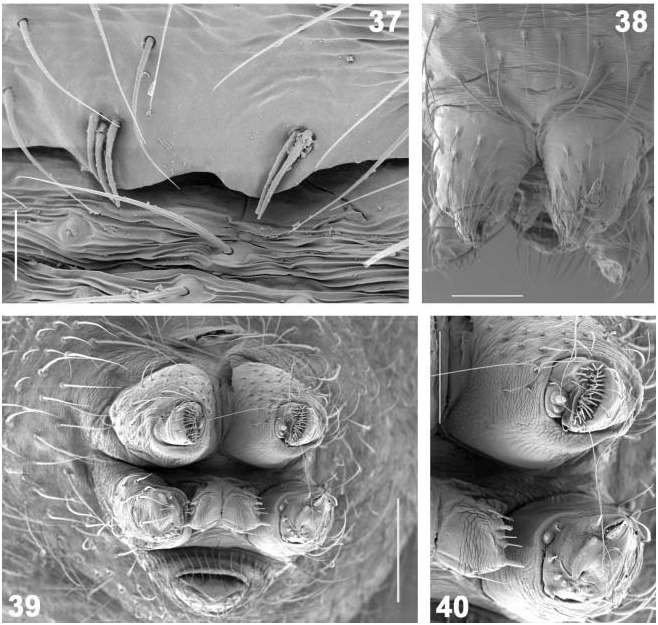
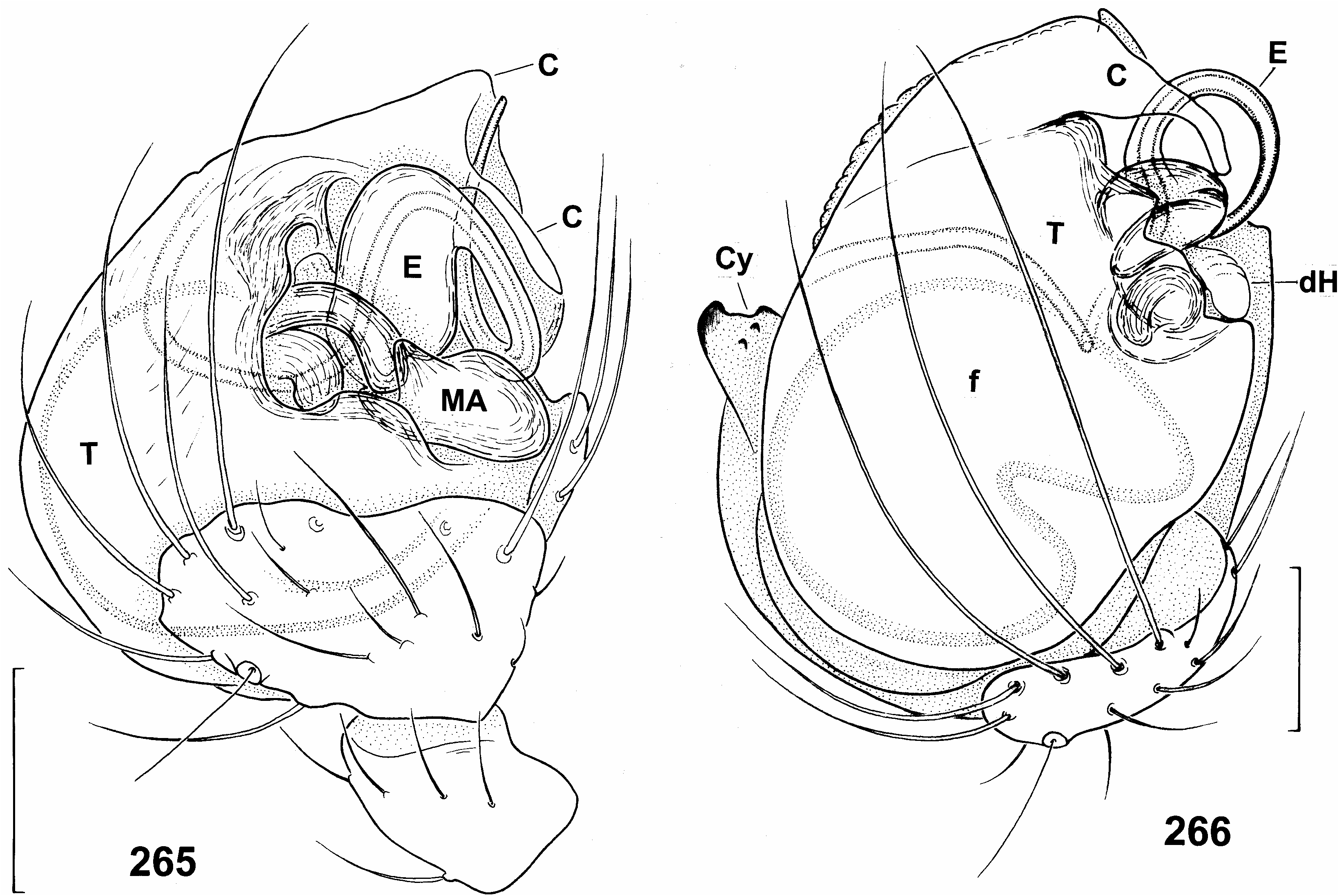
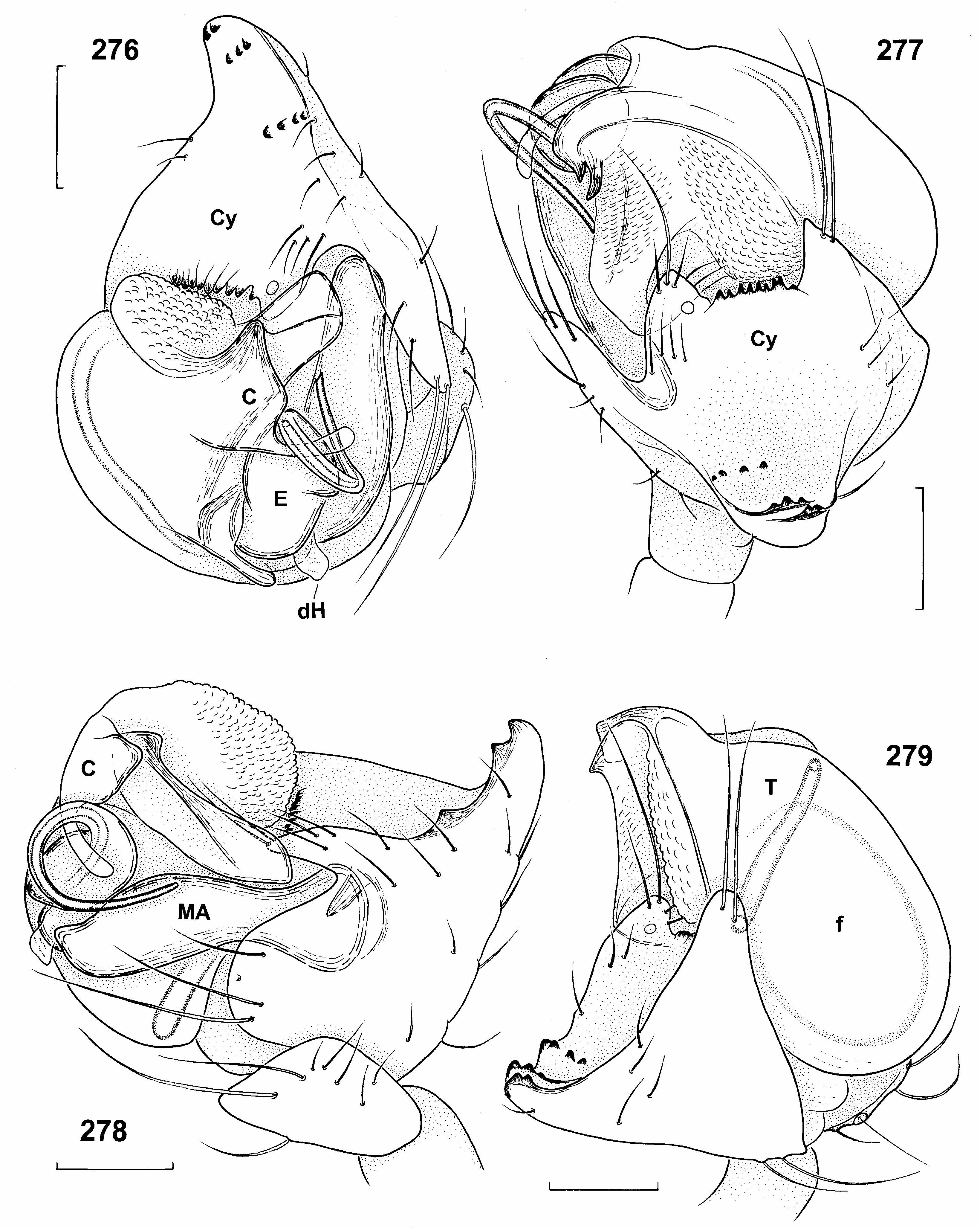
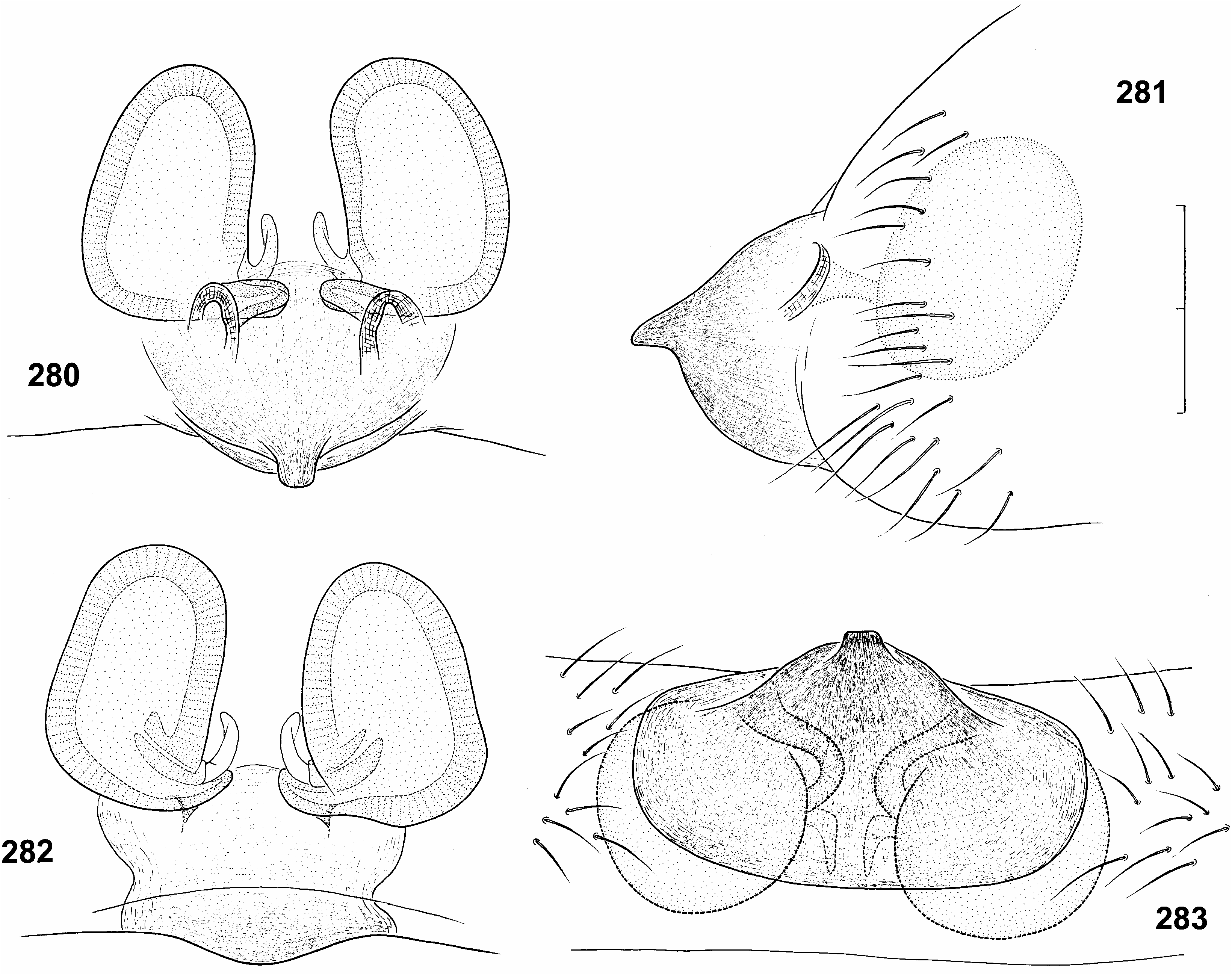
( Figures 1 View Figure 1 , 5, 6 View Figures 3–6 , 37 View Figures 37–40 , 266 View Figures 265, 266 , 276–289 View Figures 276–279 View Figures 280–283 View Figures 284, 285 View Figures 286–289 ; Tables XXXII, XXXIII)
Theridion sisyphoides Walckenaer 1842, p 321 ; n. sp., ♀.
Theridium fordum ( Keyserling 1884) , p 23, Plate 1, Figure 9 View Figures 7–10 , ♀.
Further synonyms: see Platnick (2006).
Material examined
El Salvador: 1♀ CTh, Tazamal near Santa Ana, 17 August 1977, leg. Kübelböck. Costa Rica: 1♀, MNHN AR 2909 , without exact locality, leg. Banks (sub Theridium fordum Keyserling ) . Mexico: „ ♀, Chiapas state, Tapachula, Rosario Itzapa , 6–12 October 2001, leg. S. P. Benjamin and J. A. Garcia-Ballinas. Descendants from one egg-sac were reared to adulthood in captivity in Innsbruck. Depository: see Knoflach and Benjamin (2003).
Description
Chamberlin and Ivie (1934, sub Tidarren fordum ), Levi (1957), Gruia (1977, „), and Agnarsson (2004).
Measurements (mm)
[„ / ♀, n 56/6, minimum–maximum (mean).] Total length 1.40–1.56 (1.49)/6.02–6.90 (6.54), carapace length 0.59–0.64 (0.62)/2.46–2.86 (2.71), width 0.57–0.60 (0.59)/2.23– 2.46 (2.28), length femur I 0.82–0.92 (0.89)/4.28–5.23 (4.99), tibia I 0.59–0.60 (0.60)/ 2.77–3.33 (3.22). Abdomen 0.92–1.19 (1.07)/4.28–5.39 (4.82) high, 0.78–0.92 (0.85)/ 3.80–4.28 (4.10) long, and 0.70–0.90 (0.80)/3.17–3.80 (3.55) wide. Ventral side (distance petiolus to spinnerets) 0.53–0.60 (0.57)/2.62–3.65 (2.99) long. Clypeus in male 0.20–0.23 (0.21) high, in female 0.53–0.62 (0.57). Chelicerae 0.25–0.31 (0.29)/0.82–0.98 (0.89) long. Sternum 0.39–0.43 (0.41)/1.37–1.66 (1.57) long and 0.39–0.41 (0.39)/1.13–1.29 (1.24) wide. Labium on average 0.07/0.33 long, 0.15/0.57 wide. Gnathocoxae 0.20–0.21 (0.20)/0.78–0.88 (0.83) long, 0.09–0.10 (0.09)/0.37–0.45 (0.41) wide. Femur of male palp 0.23–0.27 (0.25) long. Leg formula 1423, see Tables XXXII, XXXIII. Number of dorsal setae on tibiae I–IV 2/2/1/2. Trichobothria in retrodorsal/prodorsal row on tibia of female palp 2/2, of legs I–IV 5/2, 5/2, 4/3, 5/ 5 in female and 2/1, 2/1, 1/2, 2/ 2 in male (1 „, 1♀ examined). Metatarsi I–III with one trichobothrium, position on I in female (male) 0.31 (0.24), on II 0.33 (0.26), on III 0.37 (0.36). Tarsal claws of legs with five to six (one to two) side teeth in female (male), claw of female palp consisting of seven teeth. Tarsal organ on female palp at 0.81, on female (male) legs I–IV 0.54 (0.36), 0.45 (0.38), 0.41 (0.32), 0.48 (0.29).
Somatic features, colouration ( Figures 1 View Figure 1 , 286–289 View Figures 286–289 )
Sternum without posterior tubercle. Female sternum with three pairs of tiny lateral tubercles level with coxa I–III. Abdomen higher than long, rounded, without tubercle. In one female indistinct abdominal tubercle present. Overall colouration light brown in female, reddish in male.
Female ( Figures 1a View Figure 1 , 286–289 View Figures 286–289 ). Carapace light brown with broad dark margins and dark median band from eye region to centre, tapering posteriorly and extending laterally at centre. Clypeus greyish. Chelicerae, gnathocoxae, and labium light brown. Sternum light brown, with dark stripes opposite each coxa from margin to centre. Posterior half darker than anterior. Palps uniformly light brown, sometimes tibiae and tarsi distally darkened. Legs light brown with dark patches and annulations. Numerous fine dots on femora, tibiae and metatarsi. Coxae light brown. Abdomen light brown, with dark and some white patches. Dorsum with irregular small dark patches of various extent, sometimes outlined by white lines, which branch laterally. Apex marked by a dark patch, from where a white stripe leads down to spinnerets. Aboral region and sides light brown, with several dark spots. Epigastric region light brown, book lung covers light brown. Venter anteriorly dark, posteriorly light owing to two large white paramedian patches. Spinnerets brown.
Male ( Figures 1b View Figure 1 , 286–289 View Figures 286–289 ). Carapace light yellow to reddish brown with dark margins and dark median area from eye region to centre, tapering posteriorly. Clypeus light greyish. Chelicerae, gnathocoxae, and labium uniformly light brown. Sternum light brown, with fine dark margins. Legs uniformly yellow to reddish brown, sometimes suffused with grey. Male palp yellow brown from coxa to tibia. Distal parts of cymbium reddish brown. Abdomen uniformly reddish brown, with indistinct greyish longitudinal band, ending in apical region. Spinnerets brown.
Male palp ( Figures 266 View Figures 265, 266 , 276–279 View Figures 276–279 , 285 View Figures 284, 285 )
Very conspicuous and three-dimensional, as cymbium and bulbus face in various directions. Most noticeable part formed by highly modified cymbium ( Figures 276–279 View Figures 276–279 ). Tibia ca 0.5–0.6 width of palpal organ. Cymbium with two distal lobes, which bear long hairs and face bulbus ( Figure 285 View Figures 284, 285 ). Distal rim between two lobes with several teeth. Another large sclerotized apical projection bearing three rows of teeth faces in opposite direction. Cymbium projecting well beyond bulbus. Bulbus wider than long (0.41 wide and 0.34 mm long, n 51). Distal rim of tegulum sclerotized and bulging, but without distinct processes ( Figure 266 View Figures 265, 266 ). Retrolateral-dorsal part of conductor membranous, with hyaline, slender appendix ( Figures 276, 278 View Figures 276–279 ). Prolateral-ventral part larger, more sclerotized, its surface covered with numerous tiny scales. Embolar base suboval, with two small basal lobes (partially visible in Figure 266 View Figures 265, 266 ). Distal part of embolus 0.24 mm long. Haematodochae not modified ( Figures 1b View Figure 1 , 287 View Figures 286–289 ).
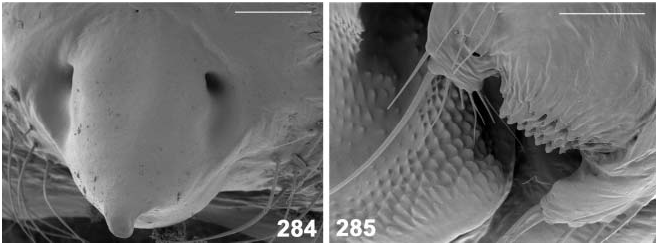
Epigynum, vulva ( Figures 280–284 View Figures 280–283 View Figures 284, 285 )
Epigynal protuberance broad at base, but ending in small pointed tip in side view ( Figures 281 View Figures 280–283 , 284 View Figures 284, 285 ). Sclerotized aboral side forms a transverse suboval plate, clearly delimited from surrounding integument ( Figure 283 View Figures 280–283 ). Copulatory orifices separate ( Figures 280 View Figures 280–283 , 284 View Figures 284, 285 ), situated paramedially at anterior border of epigynal protuberance, in ventral and dorsal view at level of posterior end of receptacula. Copulatory ducts short ( Figures 280, 282 View Figures 280–283 ), ca 0.15 mm long, turn to midline, then immediately enter receptacula at posterior inner side. Epigynal protuberance 0.2 mm long in side view, 0.3 mm wide in ventral view, almost as long as receptacula. Receptacula seminis 0.25 mm long and 0.16 mm wide.
Copulatory behaviour ( Figures 1b View Figure 1 , 286–289 View Figures 286–289 )
Tidarren sisyphoides differs from the other congeners in some respects ( Knoflach and Benjamin 2003). Courtship proceeds without construction of a mating thread and females remain motionless. The male mounts the female venter from behind and sometimes also moves forward to the female’s sternum ( Figure 286 View Figures 286–289 ), palpating her body and legs throughout. In response to these movements, the female lowers her body. Interestingly, each insertion succeeds at once on the first attempt. At the beginning of copulation the male’s legs are stretched out straight. After about 3 min his legs become contracted ( Figures 287, 289 View Figures 286–289 ), evidently indicating the male’s death. He then remains passively coupled to the female for the next 2.4 h, while she is cataleptic. There is no obvious movement by either partner. Finally, the female removes the dead male from the epigynum ( Figure 288 View Figures 286–289 ) and casts him away without cannibalizing him. The moment of removal is determined by the female. She may pull off the male after 14 min or after more than 6 h. His palp remains inflated after removal ( Figure 1b View Figure 1 ).
Natural history ( Figures 5, 6 View Figures 3–6 )
Tidarren sisyphoides occurs around buildings, below overhanging rock cliffs, trunks of trees, and under bridges ( Chamberlin and Ivie 1934; Guarisco 2000). The web has an average diameter of about 60× 30 cm, and the retreat consists of dried leaves, debris, and prey remains and forms an inverted basket. Gum-footed lines or viscid elements are absent ( Benjamin and Zschokke 2003). Nevertheless, this species is able to catch large prey, such as harvestmen, crickets, katydids ( Guarisco 2000), and in captivity they were fed with crickets and tenebrionid larvae. The egg-sacs are brown, parchment-like. Fecundity is considerable in this species, 350 and 477 eggs were found per sac ( Guarisco 2000). Postembryonic development lasts longer compared with the other Tidarren species , related to its larger size. Males moult three to four times (incomplete stages within cocoon not taken into account) and mature ca 55 days after hatching from the cocoon; females need five to six moults and ca 100 days to reach maturity ( Gonzalez 1982). The subadult stage of males reared from a Mexican egg-sac lasted 11.6 days on average (¡0.54 SE, range 10–18, n 518). Tidarren sisyphoides was the first species in which the process of palp amputation was described ( Branch 1942, sub T. fordum ; see also Figures 5, 6 View Figures 3–6 ). One amputation occurred ca 2 h after the moult and took only 3 min ( Knoflach and Benjamin 2003). Males cohabit in female webs, up to a dozen males have been found in a single web ( Chamberlin and Ivie 1934).
Enemies. In Kansas T. sisyphoides was observed to suffer severe predation by Mimetus puritanus Chamberlin, 1923 ( Mimetidae ; Guarisco 2000). Also the araneophagic theridiids Argyrodes trigonum (Hentz, 1850) and A. cancellatus (Hentz, 1850) invaded the webs of T. sisyphoides . The mantispid Mantispa viridis Walker, 1853 was encountered from egg-sacs of T. sisyphoides in Kansas ( Guarisco 2000). In Florida an egg-sac was infested by the eulophid hymenopteran Comastichus zopheros LaSalle, 1994 (see Guarisco 2001).
Distribution
Tidarren sisyphoides occurs from southern USA to South America, its northernmost locality in Kentucky ( Levi 1957), southernmost in Argentina (Catamarca; see Gonzalez 1982). However, numerous records exist from Georgia and Florida to California and Mexico, but only a few from South America. The species is also known from the West Indies ( Cuba, Haiti, and Puerto Rico; Levi 1957).
| MNHN |
Museum National d'Histoire Naturelle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Tidarren sisyphoides ( Walckenaer, 1842 )
| Knoflach, Barbara & Harten, Antonius Van 2006 |
Theridion sisyphoides
| Walckenaer CA 1842: 321 |