Terminalia kangeanensis Slooten (1924: 35)
|
publication ID |
https://doi.org/10.11646/phytotaxa.570.2.6 |
|
DOI |
https://doi.org/10.5281/zenodo.7256666 |
|
persistent identifier |
https://treatment.plazi.org/id/DB628793-FFB6-FFF9-92E2-2B4EFE4B90FC |
|
treatment provided by |
Plazi |
|
scientific name |
Terminalia kangeanensis Slooten (1924: 35) |
| status |
|
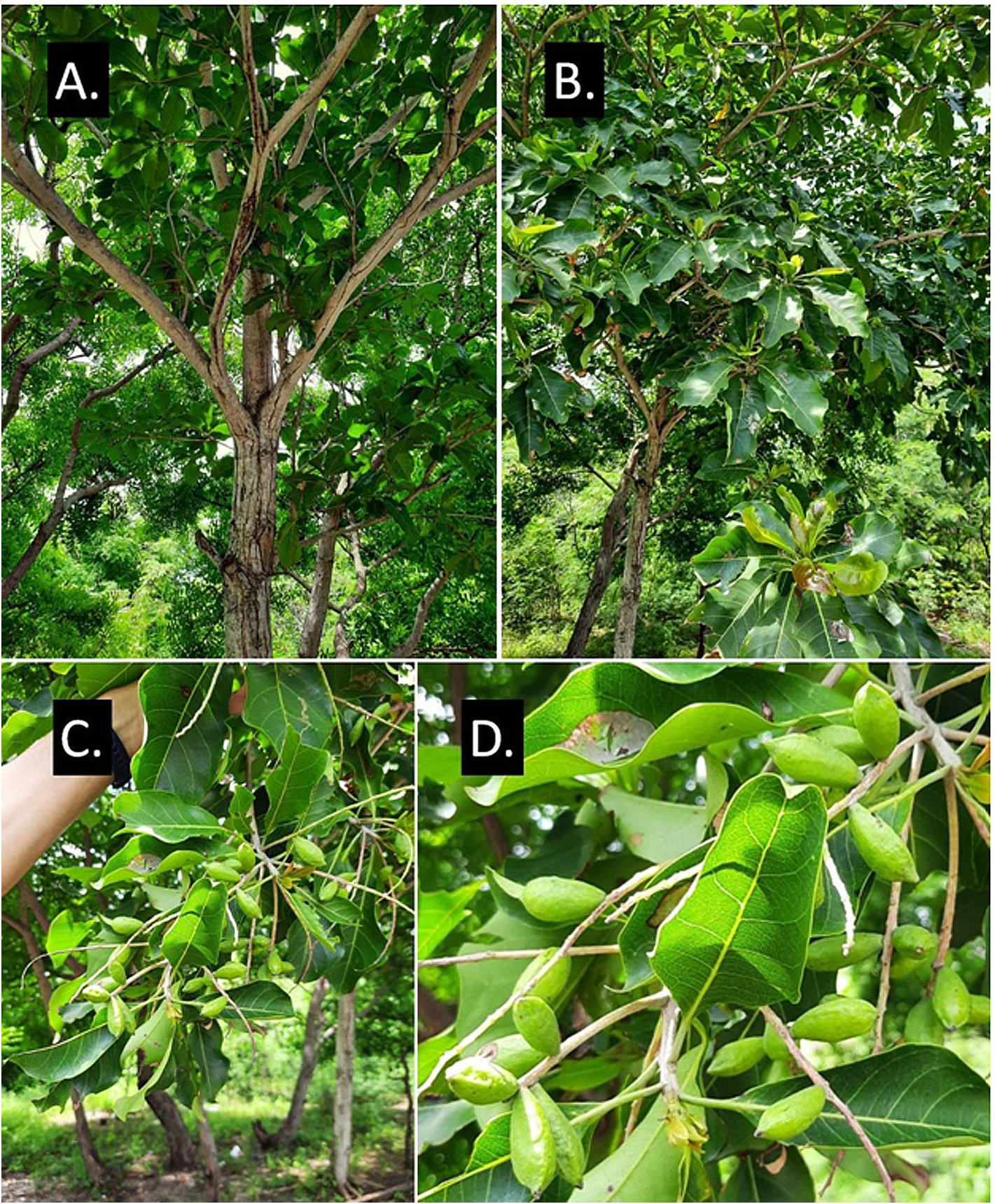
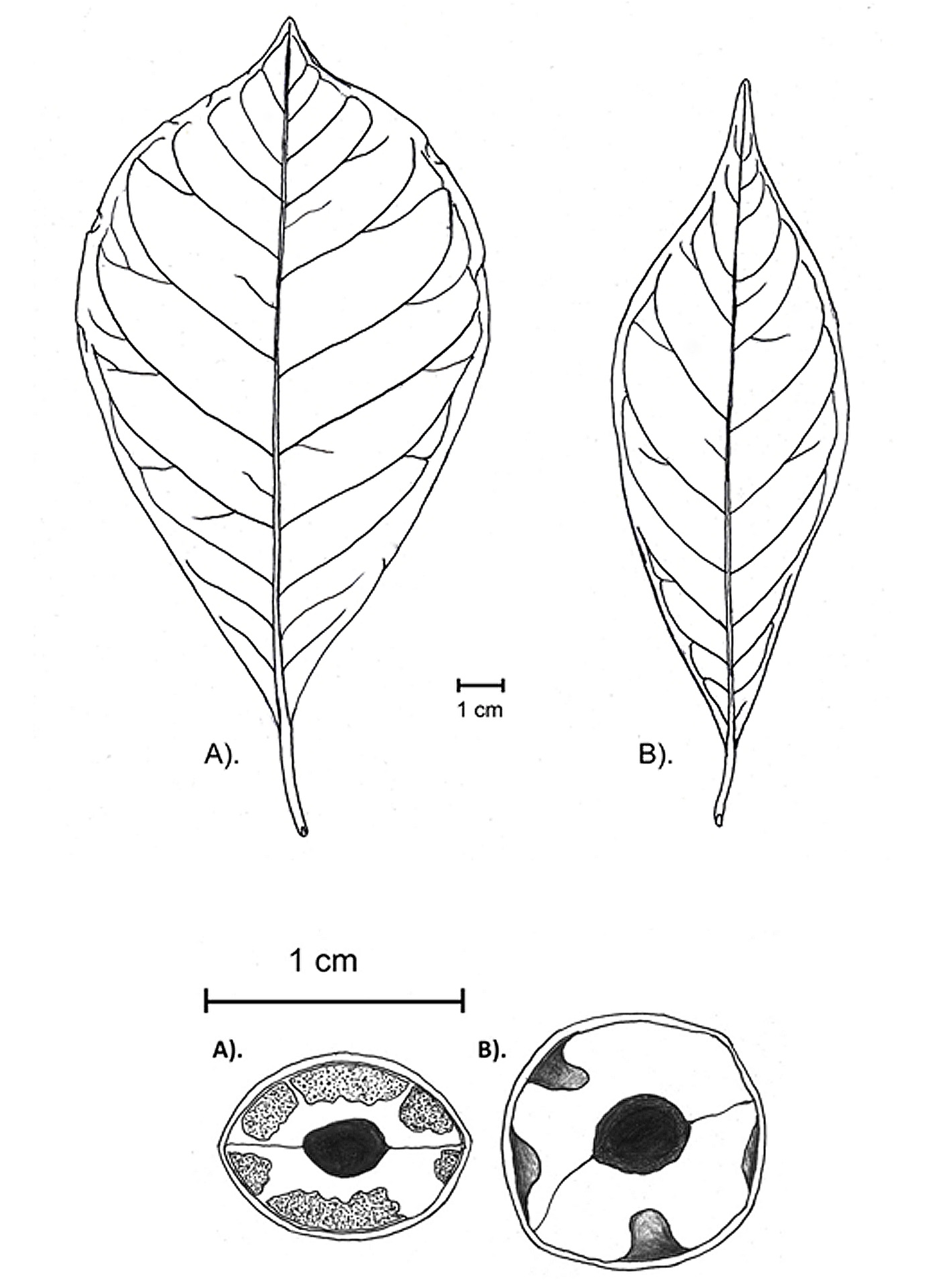
Terminalia kangeanensis Slooten (1924: 35) View in CoL . Figures 3 View FIGURE 3 , 4 View FIGURE 4 , 5 View FIGURE 5 .
Type :— INDONESIA. Kangean archipelago, Sapapan island. Grow on cliff, alt. ca. 5 m, 14 April 1919, Backer 28449, barcode L – 0867768, catalogue number L.2494369 ( L!, lectotype, designated here) . Remaining syntypes:— INDONESIA. Koorders 188, BO-1684132 ( BO!); Backer 27306, barcode L –0867769, catalogue number L.2494368 ( L!); Backer 29464, barcode L –0867767, catalogue number L.2494370 ( L!) .
Additional examined specimen:— INDONESIA. East Java, Tabuhan island. Grow on the seashore, alt. ca. 2 m, 6 February 2021, RIO 9271 ( BO!) .
Medium-sized tree, up to 7 meters tall, basal stem reaching 18 cm in diam., stem and branch light brown or light grayish-brown, bark of the old stem usually cracked longitudinally, terminal buds and young shoots densely pubescent with silvery or golden fine hairs, becoming glabrous with age. Leaves spirally and densely arranged at the top of the branchlets, pubescent when young and glabrous when mature, fresh leaves coriaceous, becoming thick chartaceous when dry, lamina obovate to broadly obovate or rarely broadly elliptic, 7.6–19.5 cm long x 3.2–9.6 cm wide, cuneate at base, apex usually shortly acuminate or sometimes obtuse, midrib and lateral-veins prominent adaxially and abaxially with obvious reticulate venation, lateral veins 8–12 at each side, margins undulate, abaxial surface with several obvious relatively large domatia in vein axils, the upper half veins of the adaxial surface usually with minutely prominent glands, lamina dark green with yellowish midrib and veins; petioles 0.9–2.7 cm long x 0.25–0.35 cm wide, densely pubescent with minute hairs especially in the basal area. Inflorescences spiciform, arising near the tip of branchlet, in axils of leaves, up to 8 spikes, each 7–15 cm long, each usually bearing more than 40 flowers, peduncle and rachis minutely pubescent. Flower not seen. Fruits obliquely ellipsoid, sub-cylindrical to slightly compressed laterally, 2.0–3.0 cm long x 0.9–1.4 cm wide, minutely pubescent when young, glabrous when mature, outer surface uneven textured, cuneate at the base, apex usually pointed (sub-acute to acuminate) or sometimes obtuse; cross-section of the fruit showing sclerenchymatous tissue surrounding the loculus with 4–6 spoke-like projections with rather large yellowish alveolar tissue lying between them.
Ecology
The trees of T. kangeanensis in Tabuhan are growing very near to the sea just about 18–25 meters from shorelines and surrounded by common plants such as Casuarina equisetifolia Linnaeus (1759: 143) , an unidentified species of Alstonia Brown (1810: 64) , Calotropis gigantea ( Linnaeus 1753: 214) W.T. Aiton (1811: 78) , and Azadirachta indica A. Jussieu (1830: 221) . Although there was a previous record of this species growing in a mixed rainforest on limestone at 50 m elev., most other records indicate that T. kangeanensis is well adapted and favored to the low elevation environments such as coastal forest ecosystems. The alveolar tissues around the seed may have a function as air-chamber, allowing the fruit or seed to float and be carried by the ocean waves over great distances across the surrounding islands.
Discussion
Careful morphological observations of the living collection labeled as T. kangeanensis in Bogor Botanical Garden (living collection XXIV.A.6) allowed us to conclude that the features of this plant do not match those of T. kangeanensis . This plant differs from T. kangeanensis in having narrowly oblanceolate to oblong elliptic leaf laminas with entire margins that usually bear a long acuminate or apiculate apex (vs. obovate to broadly obovate laminas with undulate margins and short acuminate apex in T. kangeanensis ), the abaxial leaf surface lack domatia on their vein axils (vs. several obvious relatively large glabrous domatia visible on the vein axils), leaves either fresh or dry are thintextured (vs. thick coriaceous when fresh and thick chartaceous when dry), and cross-section of the fruits show only thick sclerenchymatous tissue without any alveolar tissue lying between it (vs. cross-section of the fruit showing sclerenchymatous tissue surrounding the loculus with 4-6 spoke-like projections with rather large yellowish alveolar tissue lying between them) ( Figure 5 View FIGURE 5 ). Based on those characteristics, this cultivated plant may be tentatively assigned to T. microcarpa Decaisne (1834: 457) . Therefore, the identity of this living collection in Bogor Botanical Garden (XXIV.A.6) should be revised and no longer considered as the latest record for T. kangeanensis .
Moreover, based on their natural habitat preference, species of Terminalia can be divided into two groups: shadeadapted or sun-adapted. The plant in Bogor Botanical Garden may be categorized as a shade-adapted species which apparently prefers low or medium light intensity rather than full sun, as one could suspect from its very thin textured leaves with less developed waxy cuticle layers on the adaxial surface, in contrast with T. kangeanensis , which is a sunadapted species that prefer to grow in high light intensity/full sun habitat as well as T. catappa Linnaeus (1767: 128) .
Although floral characters are important in identifying species of Terminalia , the combination of characters of leaf and fruit (including seed) are often seen as more reliable for identification at species level ( Exell 1954; Goode 1969; Nanakorn 1985: Gangopadhyay & Chakrabarty 1992; Anozie 1995; Chakrabarty et al. 2019). The careful examinations to the specimens collected from Tabuhan island in 2021 have shown that the leaf and fruit morphological characteristics matched well the protologue, type specimens, and line drawings of Terminalia kangeanensis in terms of their size and shape. Therefore, the discovery of living specimens of T. kangeanensis in Tabuhan island can be considered as a rediscovery, as well as a new record for its natural distribution (150–160 kilometers away from previously known habitats). This paper also provides additional details on its colour, morphological characters, and ecological information to complement the previous descriptions. The photos of living T. kangeanensis in this paper probably are the first photographs that ever be published.
All currently known habitats of T. kangeanensis (including Tabuhan) are categorized as small islands. Among these four islands, two of them (Tabuhan and Mamburit) only just about 6-8 ha, another one is 157 ha (Sapapan), and the largest is just 4500 ha (Paliat). Tabuhan is an uninhabited island but heavily visited for tourism, while Mamburit has about 50 families and some areas of this island are submerged during the high tide. Tabuhan was officially leased in 2020 to a foreign investor, and about 65% of the island is planned for private areas with additional infrastructure development ( Sedana 2020). Sapapan is an inhabited land where more than half of the island has been converted for settlements and seasonal agriculture, leaving only narrow coastal forests along the shore in its northern part. Likewise with Paliat, where most of the lands have turned into large open areas and leaving just a very narrow green belt along the shore with scattered and fragmented small forests.
There is a possibility to find other natural populations outside previously known localities by considering several aspects, such as the distance between currently known habitats, the direction of ocean surface currents circulation for seed dispersion, and the natural vegetation coverage. Therefore, the authors predicts that T. kangeanensis may also be found along the most northeastern coast of East Java (these including Baluran National Park in Situbondo Regency and the northern part of Banyuwangi Regency) and also along the coast of the West Bali National Park in Bali island. Of course this hypothesis has to be confirmed through direct field survey.
It has been widely known that species or populations in the small island are fragile and more prone to extinction ( Simberloff 2000; González-Mancebo et al. 2012). This is due to isolation that limits their living area ( Gillespie et al. 2008), higher interaction with human-induced disturbances (habitat conversion and invasive species; Duncan & Blackburn 2007), and the tendency to high risk of genetic drift and inbreeding depression in small size populations ( Escobar et al. 2008). The ongoing anthropogenic climate change may exacerbate the impact of those threats, making future conservation efforts even more challenging ( Benning et al. 2002; Wood et al. 2017; Metusala et al. 2017). Then it would make sense to promote conservation priority for threatened plant species that are endemic to small islands, particularly those already exposed to high levels of disturbance.
Both taxonomic study and botanical exploration of the genus Terminalia in Indonesia are urgently in need to be held intensively to provide a better picture of its diversity and conservation status. The botanists are in the race with rapid land conversion rates of the lowland ecosystems. Therefore, research priority should be given before they are all gone in the near future.
| L |
Nationaal Herbarium Nederland, Leiden University branch |
| BO |
Herbarium Bogoriense |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Terminalia kangeanensis Slooten (1924: 35)
| Metusala, Destario & Farishy, Dee Dee Al 2022 |
Terminalia kangeanensis
| Slooten, D. F. 1924: ) |