Eigenmannia catira, Cardoso & Dutra, 2023
|
publication ID |
https://doi.org/10.1590/1982-0224-2023-0090 |
|
publication LSID |
lsid:zoobank.org:pub:E356A407-A29B-4CD4-BDD1-61724DA61940 |
|
DOI |
https://doi.org/10.5281/zenodo.11086065 |
|
persistent identifier |
https://treatment.plazi.org/id/D4147509-2B2A-FF9B-FCE6-DDCBFB7FFA5B |
|
treatment provided by |
Felipe |
|
scientific name |
Eigenmannia catira |
| status |
sp. nov. |
Eigenmannia catira , new species
urn:lsid:zoobank.org:act:
( Figs. 1–8 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 ; Tab. 2 View TABLE 2 )
Eigenmannia trilineata (non López & Castello, 1966). — Pereira et al., 2013:16 (additional file, barcode, voucher LBP 4653). — Frantine-Silva et al., 2015:1232 (barcode, no voucher available).
Eigenmannia sp. — Sene et al., 2014:303 (karyotype, voucher LBP 12304).
Eigenmannia virescens (non Valenciennes, 1836). — Tagliacollo et al., 2016:26 (phylogenetic study, voucher LBP 9671).
Holotype. MZUSP 129263, 132.7 mm LEA, CT, córrego do Schmidt , tributary of rio Grande, upper rio Paraná basin, Santa Albertina, São Paulo, Brazil, 20°01’30”S 50°43’47”W, 30 Nov 2016, M. Cardoso Junior. GoogleMaps
Paratypes. All from Brazil, upper rio Paraná basin. Mato Grosso do Sul State: LBP 2647 (6, 85.7–135.3 mm LEA), rio Baia, Bataiporã, 22°43’03.2”S 53°17’27.6”W, 5 Mar 2005, R. Teixeira, E. Martinez, G. França & L. Paiva. GoogleMaps LBP 3103 (7, 75.4–115.3 mm LEA), rio Baia , Bataiporã, 22°43’19.4”S 53°17’11.3”W, 13 Oct 2005, R. Teixeira, G. França & L. Paiva. GoogleMaps LBP 4653 (15+2 CS, 78.4–85.3 mm LEA), rio Baia , Taquarussu, 22°43’46.2”S 53°19’04.2”W, 10 May 2007, R. Devidé, L. Paiva, E. Martinez & V. Cruz. GoogleMaps LBP 9621 (4, 86.9–106.7 mm LEA), riacho de Engano, Angélica, 22°02’37.5”S 53°43’38.8”W, 19 Jun 2010, R. Devidé, M. Pazian, F. Roxo & G. Silva. GoogleMaps LBP 9650 (27+3 CS, 53.6– 97.5 mm LEA), rio Ivinhema , Angélica, 22°02’52.3”S 53°41’38.1”W, 20 Jun 2010, R. Devidé, M. Pazian, F. Roxo & G. Silva. GoogleMaps LBP 9671 (3, 57.0– 77.7 mm LEA), unamed stream, Ivinhema, 22°15’29.5”S 53°48’38.7”W, 20 Jun 2010, R. Devidé, M. Pazian, F. Roxo & G. Silva. GoogleMaps LBP 9773 (12, 76.6–137.8 mm LEA), rio Papagaio , Nova Andradina, 21°53’48.1”S 53°47’25.1”W, 22 Jul 2010, R. Devidé, M. Pazian, F. Roxo & G. Silva. GoogleMaps MZUSP 88455 View Materials ( 1, 126.4 mm LEA), córrego do Palmito below bridge of MS-262 road, near Fazenda Santa Ângela, Três Lagoas, 20°49’57.67”S 51°47’55.20”W, 24 Sep 2003, O. Oyakawa, J. Birindelli & C. Kikuchi. GoogleMaps Paraná State: NUP 12450 (7, 63.0– 80.9 mm LEA), Ressaco do Bilé at Ilha Mutum , Porto Rico, 22°45’13”S 53°17’09”W, 8 Oct 2011, F. Souza. GoogleMaps São Paulo State: LBP 12304 ( 1, 123.2 mm LEA), rio Hortelã , Botucatu, 22°55’23.2”S 48°32’40.4”W, 27 Jan 2011, R. Devidé, J. Alves & R. Utsonomia. GoogleMaps MZUSP 83398 View Materials (3, 168.4– 217.2 mm LEA), rio Tietê below UHW Bariri, Bariri, 22°08’50”S 48°45’06”W, 3 Nov 2003, A. Akama. GoogleMaps MZUSP 102856 View Materials ( 1, 136.5 mm LEA), rio do Peixe, Ribeirão dos Índios, 21°41’59”S 51°31’32”W, 14 Oct 2008, P. Hollanda-Carvalho & R. Caires. GoogleMaps MZUSP 121047 View Materials (20+3 CS, 86.1–103.2 mm LEA), collected with the holotype. GoogleMaps
Non-types. All from Brazil, upper rio Paraná basin. Mato Grosso do Sul State: INPA 35360 View Materials ( 1, 109.6 mm LEA), córrego Piravevê, tributary of rio Ivinhema , Angélica , 22°13’59”S 53°47’02”W, 17 Dec 2010, M. Rocha & T. Carvalho. GoogleMaps LBP 9664 (1, 47.2 mm LEA), unnamed stream, Angélica, 22°13’59.9”S 53°47’02.6”W, 20 Jun 2010, R. Devidé, M. Pazian, F. Roxo & G. Silva. GoogleMaps MCP 45909 View Materials (2, 64.0– 87.6 mm LEA), rio Guiraí , Novo Horizonte do Sul , 22°38’23.9”S 54°00’25.3”W, 16 Dec 2010, M. Rocha. GoogleMaps MCP 45940 View Materials (2, 87.4–98.1 mm LEA), rio Guiraí , Novo Horizonte do Sul , 22°40’22”S 53°51’39”W, 16 Dec 2010, M. Rocha. GoogleMaps MZUEL 13379 ( 1, 102.5 mm LEA), rio Verde , Brasilândia, 21°02’14”S 52°08’46”W, 19 Jan 2011, P. da Silva. GoogleMaps MZUEL 14488 (43, 26.1– 126.6 mm LEA), córrego Piraveve, tributary of rio Ivinhema , Angélica, 22°14’19.3”S 54°04’08.8”W, 13 Jan 2016, M. Rocha, J. Birindelli, F. Jerep, E. Santana, Y. Suarez & F. Ferreira. GoogleMaps NUP 3201 (4, 70.5–138.0 mm LEA), lagoa do Guaraná, Taquarussu, 22°43’S 53°19’W, 20 Sep 2003, H. Júlio Jr. GoogleMaps NUP 10713 (3, 29.3–142.7 mm LEA), lagoa Ventura, Taquarussu, 22°51’24”S 53°36’01”W, 18 Nov 2004, NUPELIA team. GoogleMaps NUP 12674 (8, 99.2–159.3 mm LEA), lagoa Finado Raimundo, tributary of rio Ivinhema , Taquarussu, 22°47’57”S 53°32’29”W, 18 Nov 2004, NUPELIA team. GoogleMaps NUP 12687 (7, 94.7–121.8 mm LEA), lagoa dos Patos, tributary of rio Ivinhema , Taquarussu, 22°49’33”S 53°33’09”W, 16 Nov 2006, NUPELIA team. GoogleMaps NUP 12702 (1, 143.1– 170.1 mm LEA), lagoa Finado Raimundo, tributary of rio Ivinhema , Taquarussu, 22°47’57”S 53°32’29”W, 17 Dec 2007, NUPELIA team. GoogleMaps NUP 12713 (2, 115.5– 110.5 mm LEA), GoogleMaps NUP 12736 ( 1, 116.3 mm LEA), lagoa dos Patos, tributary of rio Ivinhema , Taquarussu, 22°49’33”S 53°33’09”W, 17 Dec 2007, NUPELIA team. GoogleMaps NUP 12723 (23, 67.6–104.4 mm LEA), lagoa dos Patos, tributary of rio Ivinhema , Taquarussu, 22°49’33”S 53°33’09”W, 20 Jun 2005, NUPELIA team. GoogleMaps NUP 12724 (1, 93.8 mm LEA), GoogleMaps NUP 12751 (3, 67.3– 74.7 mm LEA), lagoa dos Patos, tributary of rio Ivinhema , Taquarussu, 22°49’33”S 53°33’09”W, 23 Aug 2005, NUPELIA team. GoogleMaps NUP 12735 (3, 82.1–100.5 mm LEA), lagoa dos Patos, tributary of rio Ivinhema , Taquarussu, 22°49’33”S 53°33’09”W, 16 Nov 2006, NUPELIA team. GoogleMaps NUP 12760 (1, 88.0 mm LEA), lagoa dos Patos, tributary of rio Ivinhema , Taquarussu, 22°49’33”S 53°33’09”W, 13 Feb 2008, NUPELIA team. GoogleMaps NUP 12767 (2, 70.9–82.9 mm LEA), lagoa Finado Raimundo, tributary of rio Ivinhema , Taquarussu, 22°47’57”S 53°32’29”W, 26 Jul 2005, NUPELIA team. GoogleMaps Minas Gerais State: NUP 14027 (2, 129.6–134.0 mm LEA), rio Paranaíba , Tupaciguara, 18°21’21”S 48°40’52”W, 16 Jul 1974, J. Silva, I. Rocinski & D. Marzulo. GoogleMaps Paraná State: NUP 362 ( 1, 110.5 mm LEA), ribeirão São Pedro, São Pedro do Paraná, 22°45’S 53°15’W, Mar 1991, C. Pavanelli et al. GoogleMaps NUP 7876 (2, 91.3– 85.3 mm LEA), rio Paracaí , São Jorge do Patrocínio , 23°39’01”S 53°56’36”W, 14 Jul 2009, NUPELIA team. GoogleMaps NUP 7878 ( 1, 140.8 mm LEA), rio Paracaí , São Jorge do Patrocínio , 23°39’24”S 53°54’23”W, 13 Jul 2009, NUPELIA team. GoogleMaps NUP 7913 (2, 112.5– 138.1 mm LEA), rio Paracaí , São Jorge do Patrocínio , 23°39’30”S 53°55’10”W, 13 Jul 2009, NUPELIA team. GoogleMaps NUP 7918 (2, 103.4– 117.8 mm LEA), rio Paracaí , São Jorge do Patrocínio , 23°39’30”S 53°55’10”W, 13 Jul 2009, NUPELIA team. GoogleMaps NUP 7939 (3, 91.4– 90.8 mm LEA), rio Paracaí , São Jorge do Patrocínio , 23°39’01”S 53°56’36”W, 14 Jul 2009, NUPELIA team. GoogleMaps NUP 14371 (7, 110.8– 124.3 mm LEA), Ressaco do Leopoldo , Porto Rico, 22°45’24”S 53°16’08”W, 5 Dec 2004, H. Júlio Jr. GoogleMaps São Paulo State: MZUSP 24563 View Materials (1, 90.8 mm LEA), fazenda Edgardia, Botucatu, 22°53’S 48°27’W, 27 Sep 1974, F. C. M. B. Botucatu. GoogleMaps
Diagnosis. Eigenmannia catira , a member of the E. trilineata species-group ( sensu Dutra et al., 2021), differs from E. macrops and the E. humboldtii species-group by the presence of lateral line stripe ( vs. absence). The new species is further distinguished from E. macrops by the eye diameter corresponding to 14.5–24.5% HL ( vs. 26.4–29.7% HL), and the caudal filament corresponding to 13.3–41.9% LEA ( vs. 67.5–79.3% LEA). It can be further distinguished from the E. humboldtii species-group by the anal fin hyaline ( vs. anal-fin margin distinctly darkened).
Eigenmannia catira differs from all other species of the E. trilineata species-group, except E. antonioi Peixoto, Dutra & Wosiacki, 2015 , E. cacuria Dutra, Ramos & Menezes, 2022 , E. desantanai Peixoto, Dutra & Wosiacki, 2015 , E. guairaca , E. lorenata Waltz & Albert, 2018 , E. magoi Herrera-Collazos, Galindo-Cuervo, Maldonado-Ocampo & Rincón-Sandoval, 2020 , E. matintapereira Peixoto, Dutra & Wosiacki, 2015 , E. microstoma (Reinhardt, 1852) , E. muirapinima Peixoto, Dutra & Wosiacki, 2015 , E. pavulagem Peixoto, Dutra & Wosiacki, 2015 , E. sayona Peixoto & Waltz, 2017 , E. trilineata , and E. zenuensis Herrera-Collazos, Galindo-Cuervo, Maldonado-Ocampo & Rincón-Sandoval, 2020 , by having a terminal mouth ( vs. subterminal). The new species differs from the aforementioned species by the following combination of characters: (1) lateral line stripe extending from first perforated lateral line scale to distal portion of caudal filament ( vs. lateral line stripe restricted to last two thirds of body in E. cacuria ); (2) superior midlateral stripe absent ( vs. present in E. antonioi , E. cacuria , E. desantanai , E. guairaca , E. lorenata , E. magoi , E. microstoma , E. muirapinima , E. pavulagem , E. sayona , E. trilineata , and E. zenuensis ); (3) ii,12–14 pectoral-fin rays ( vs. ii, 16–17 in E. matintapereira ); (4) 174–209 anal-fin rays ( vs. 151–170 in E. guairaca , and 216–222 in E. matintapereira ); (4) 13–22 premaxillary teeth ( vs. 8–12 in E. antonioi , 24–25 in E. desantanai , 9–10 in E. guairaca , 32 in E. magoi , 8–10 in E. muirapinima , 31–33 in E. trilineata , and 31–34 in E. zenuensis ); (6) 10–19 dentary teeth ( vs. 21–23 in E. desantanai , 35–39 in E. magoi , 25–27 in E. matintapereira , 23 in E. trilineata , and 56–60 in E. zenuensis ); (7) 7–12 endopterygoid teeth ( vs. 14–15 in E. desantanai , and 16–17 in E. trilineata ); (8) all dentary teeth similar in size ( vs. dentary teeth increasing in size along dentigerous surface in E. antonioi , E. cacuria , E. loretana , E. muirapinima , E. pavulagem , and E. sayona ); (9) basibranchial 1 unossified ( vs. ossified in E. sayona ); and (10) 13–14 precaudal vertebrae ( vs. 15 in E. guairaca ). Eigenmannia catira can also be diagnosed from E. dutrai and E. guairaca by having 2n = 36 ( vs. 2n = 38 or 2n = 38, XY in E. dutrai , and 2n = 28 or 2n = 31/32–X 1 X 1 X 2 X 2 -X 1 X 2 Y in E. guairaca – data from Sene et al., 2014: tab. 1, see discussion).
Barcoding divergence. The overall mean distance among Eigenmannia species from Paraná basin was 10.1%. The lowest genetic distance between E. catira and any other Eigenmannia species from rio Paraná was 4.9% (from E. dutrai and E. virescens ), followed by 13.9% from E. guairaca , and 15.2% from E. trilineata ( Tab. 3 View TABLE 3 ).
Description. Body shape and pigmentation are shown in Fig. 1 View FIGURE 1 , and the morphometric data in Tab. 2 View TABLE 2 . Largest examined specimen 222.2 mm LEA. Body elongate and distinctly compressed laterally. Greatest body depth at vertical through distal tip of pectoral fin. Dorsal profile of body slightly convex from snout tip to vertical through anal-fin terminus. Ventral profile of body slightly convex from mandibular symphysis to end of anal fin. Caudal filament short.
Head laterally compressed, greatest width at opercular region, greatest depth at nape. Dorsal profile of head convex from snout tip to nape. Ventral profile of head slightly convex from tip of lower jaw to isthmus. Snout rounded in lateral view. Mouth terminal. Mouth rictus at vertical through a point between anterior and posterior nares or vertical through posterior nostril. Anterior nostril tube-like, closer to snout tip than to anterior margin of eye. Posterior nostril round, closer to anterior margin of eye than to snout tip. At horizontal line, posterior nostril at horizontal through dorsal margin of eye or slightly above. Eye small, circular, completely covered by skin, on anterior one-half of HL, laterally oriented. Branchial membranes joined at isthmus. Anus adjacent to urogenital papilla, shifting ontogenetically from vertical through posterior to anterior margin of opercle. Urogenital papilla not developed in specimens under 103.2 mm LEA.
Scales cycloid and small, extending from posterior most part of head to vertical through tip of caudal filament, present on mid-dorsal region of body. Scales above lateral line at vertical through end of pectoral fin 9(1), 10*(4), 11(2), 12(5), or 13(5). Anterior most perforated lateral-line scale along vertical through pectoral-fin origin. Lateral-line scales to vertical through base of last anal-fin ray 107–136(n = 25; 113*). Pectoral-fin rays ii,12(5), ii,13*(11), ii,14(9), or ii,15(1). Distal pectoral-fin margin straight. Total anal-fin rays 174–209(n = 20; 179*). Anal-fin origin along vertical through pectoral-fin insertion or slightly posterior. Distal margin of anal fin slightly convex. First unbranched rays tiny, subsequent rays progressively increasing in size toward first branched rays. Branched rays of nearly equal length except for posterior most rays that progressively decrease in length.
Coloration in alcohol. Body ground coloration cream. Body with two layers of chromatophores. Outer layer covered by dark chromatophores gradually more spaced ventrally. Chromatophores more concentrated on perforated scales forming lateral line stripe. Lateral line stripe tapering from first perforated scale until end of caudal filament. Superior midlateral stripe absent. Inner layer of pigmentation formed by multiple, small bars of dark chromatophores situated between the musculature associated with anal-fin pterygiophores. Dark individual bars in combination forming two stripe-like patterns. Inferior midlateral stripe inconspicuous, tapering from vertical through tip of pectoral-fin when adpressed on body to last anal-fin pterygiophore. Anal-fin base stripe conspicuous approximately one third as depth as orbital diameter, tapering from vertical through tip of pectoral-fin when adpressed on body to last anal-fin pterygiophore. Head covered by dark chromatophores, more concentrated on dorsal region gradually more spaced ventrally. Pectoral and anal fins hyaline with scattered dark chromatophores overlying fin rays.
Jaws. Premaxilla somewhat retangular-shaped, relative length of premaxilla twice as long as its width. Anterolateral process on premaxilla about one third of anterior margin of bone. Premaxilla with 13(1), 14(1), 17*(2), 18(1), 20(2), 21(1), or 22(2) villiform teeth arranged in two (2), or three*(7) rows. Teeth firmly, but not fully ankilosed, attached to ventral surface of premaxilla. Maxilla edentulous, slender, and slightly curved posteriorly. Descending blade of maxilla narrower than its dorsal most portion. Anterior hook-like process on maxilla present. Cartilaginous autopalatine connecting posterior cartilage of maxilla to endopterygoid.
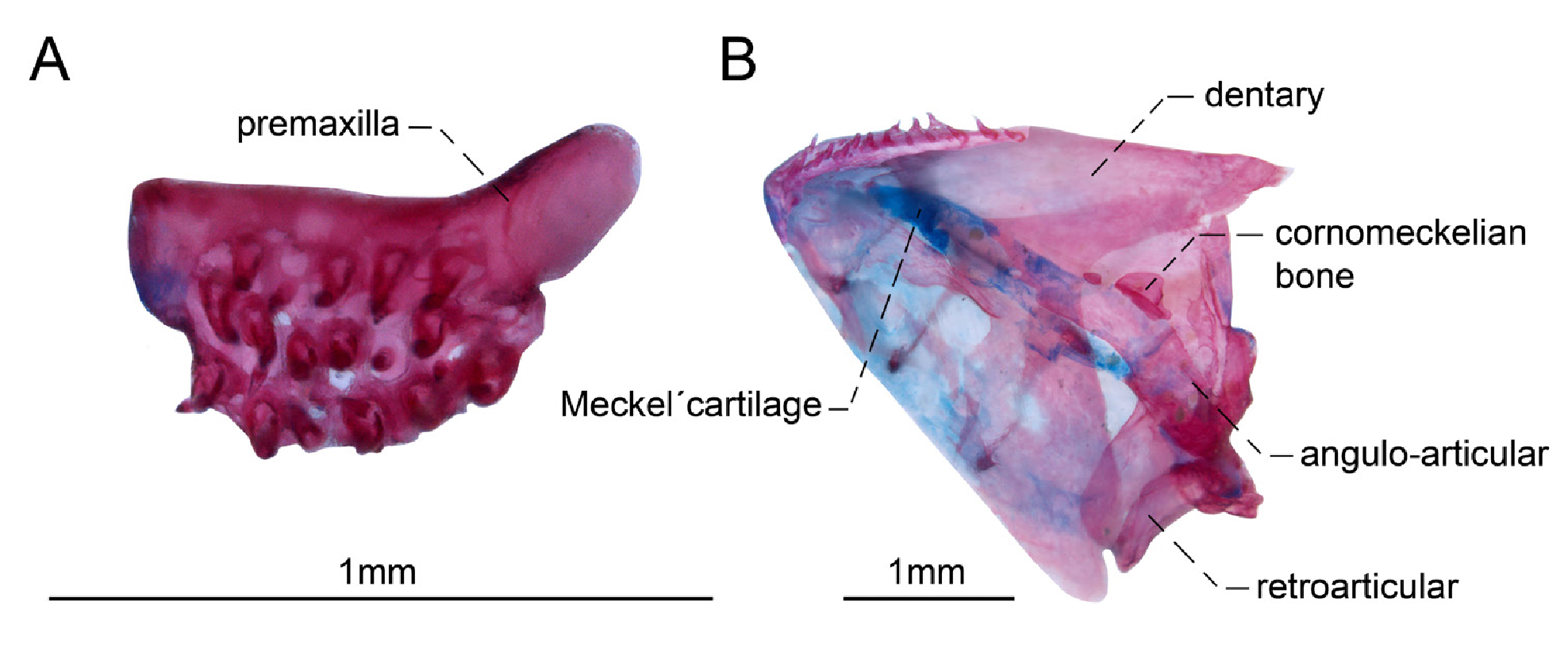
Dentary as long as lower jaw depth, with 10(2), 12(1), 15(1), 16(2), 17(2), or 19*(2) villiform teeth arranged in one (3), or two*(6) rows. All teeth similar in size along dentigerous surface, which extends beyond anterior limit of Meckel’s cartilage. Coronoid process ossified, ventrally curved in its posterior end. Meckel’s cartilage rectangular shaped, its anterior portion approximately as wide as posterior portion. Coronomeckelian bone 20% of length of Meckel’s cartilage. Angulo-articular with a narrow crest on posterolateral surface. Retroarticular small, roughly rectangular, located at posteroventral margin of anguloarticular. Retroarticular not included in socket that receives condyle of quadrate ( Figs. 2–3 View FIGURE 2 View FIGURE 3 ).
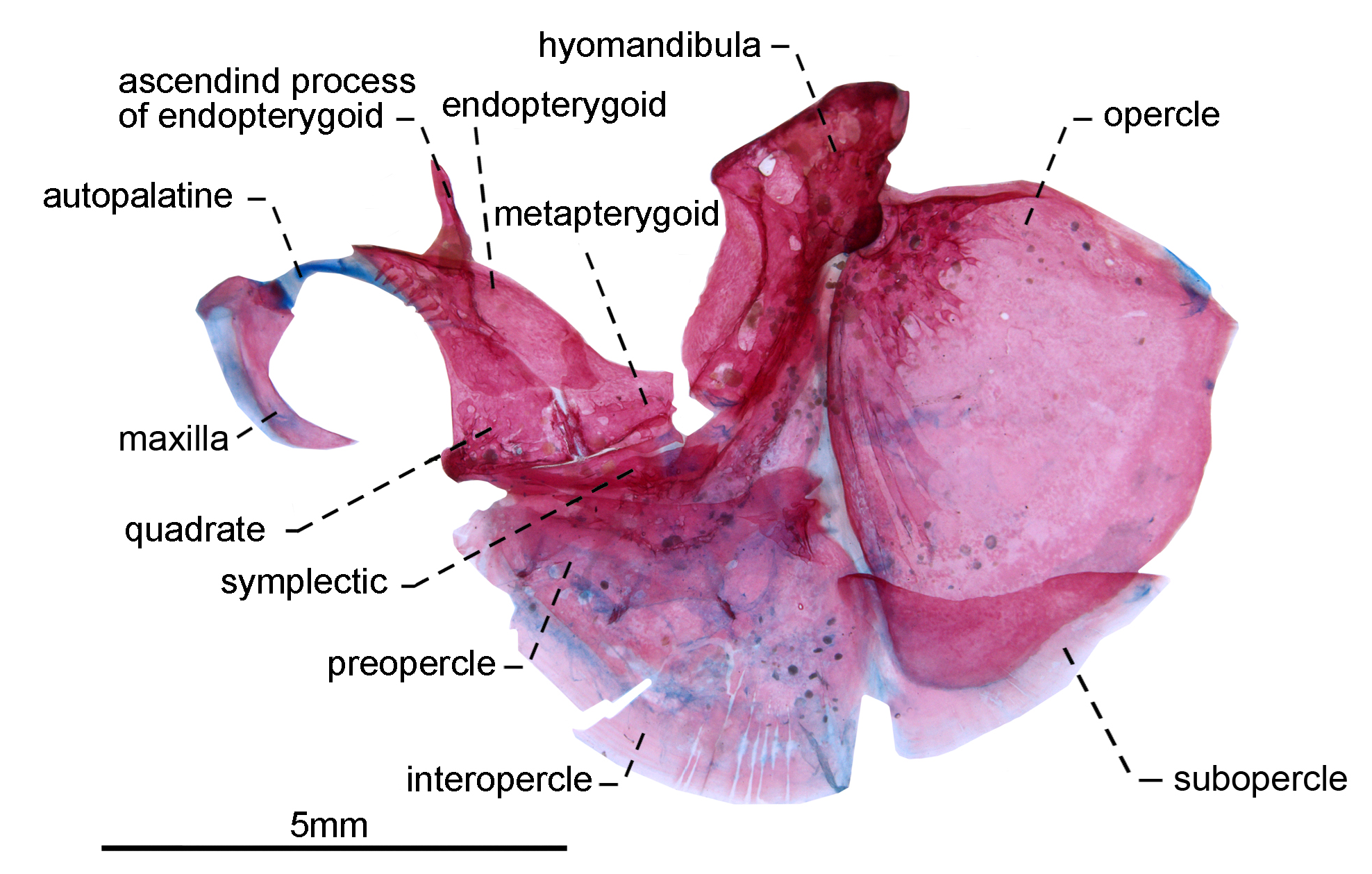
Suspensorium and opercular apparatus. Endopterygoid roughly triangular with well-developed dorsally directed process, half as long as its length, attached to anterior portion of orbitosphenoid. Relative length of anterior portion of endopterygoid, as long as its depth. Ventral surface of endopterygoid with small, pointed, conical teeth on arranged in one (6), or two (2) rows at anterior portion. Endopterygoid teeth seven (3), eight (1), nine*(4), 11(1) or 12(2). Quadrate roughly trapezoidal in shape, with a posteroventral process that articulates with preopercle and symplectic; its condyle extending anteroventrally from base and articulating with anguloarticular ( Fig. 3 View FIGURE 3 ). Metapterygoid slightly trapezoidal, relative length of metapterygoid as long as its largest depth. Symplectic elongate and triangular, located in a medial crest of preopercle and posteroventral portion of quadrate. Relative length of symplectic two thirds as long as hyomandibula. Hyomandibula at roughly 90° to horizontal line through long axis of head. Neurocranial articulatory head roughly three times wider than ventral margin. Laminar anterior shelf from widest hyomandibula point to anteroventral margin. Main axis of hyomandibula forming an angle of nearly 40° in relation to its dorsal margin. Condyle that receives the opercular distinct from main body of hyomandibula and directed posteroventrally. Posterodorsal portion of hyomandibula extending posteriorly to condyle that receives opercular socket. Posterodorsal margin of hyomandibula with a large foramen in which recurrent ramus of the anteroventral part of the anterior lateral line nerve passes before extending to body.
Preopercle crescent-shaped with five bony arches corresponding to laterosensory canal along its lateral surface. Interopercle fan-shaped, with posterodorsal expansion and margins rounded. Opercle roughly triangular, dorsal margin convex, with a pointed anterodorsal process. Subopercle sickle-shaped, tapering posterodorsally, forming concave dorsal profile. Subopercle and interopercle becoming membranous distally.
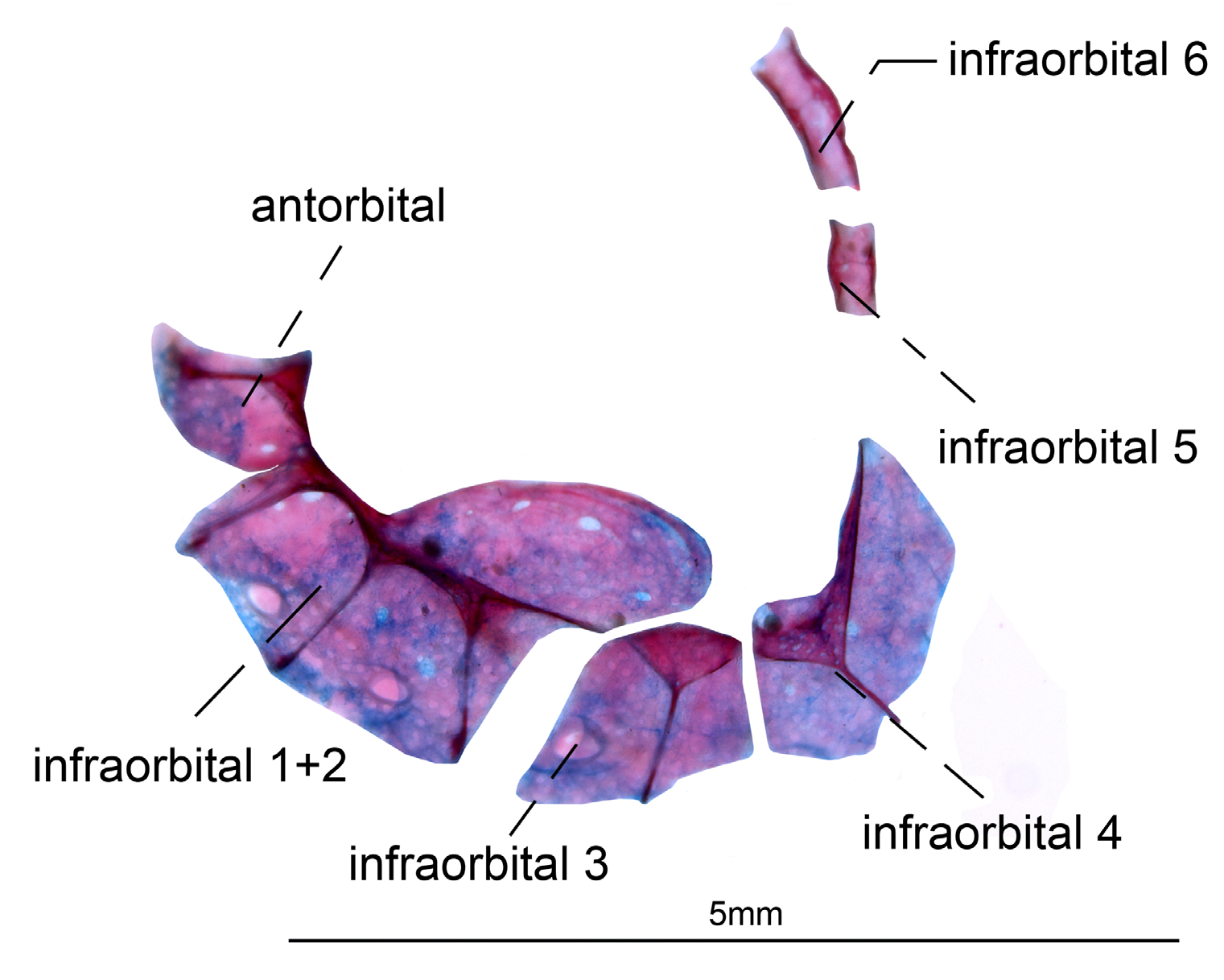
Cephalic lateral line system. Canals of nasal, antorbital and infraorbitals 1+2 to 4 enlarged and half-pipe shaped. Infraorbitals 1+2 with three bony arches. Depth of posterodorsal laminar expansion of infraorbitals 1+2 half as long as its length. Infraorbitals 3 and 4 closely associated. Fifth and sixth infraorbitals slender and tubular. Mandibular canal and preopercular canals enlarged and half-pipe shaped. Mandibular canal with three poorly ossified bony arches fused along lateroventral surface of dentary. Preopercular with four ossified bony arches. Supraorbital canal robust, forming a highly ossified shelf-like structure fused to frontal bone. Connection between infraorbital and supraorbital canal on sphenotic process. Otic and extrascapular canals slender and tubular shaped. Extrascapular slender and tubular located on joint of parietal, pterotic and epioccipital ( Figs. 4–5 View FIGURE 4 View FIGURE 5 ).
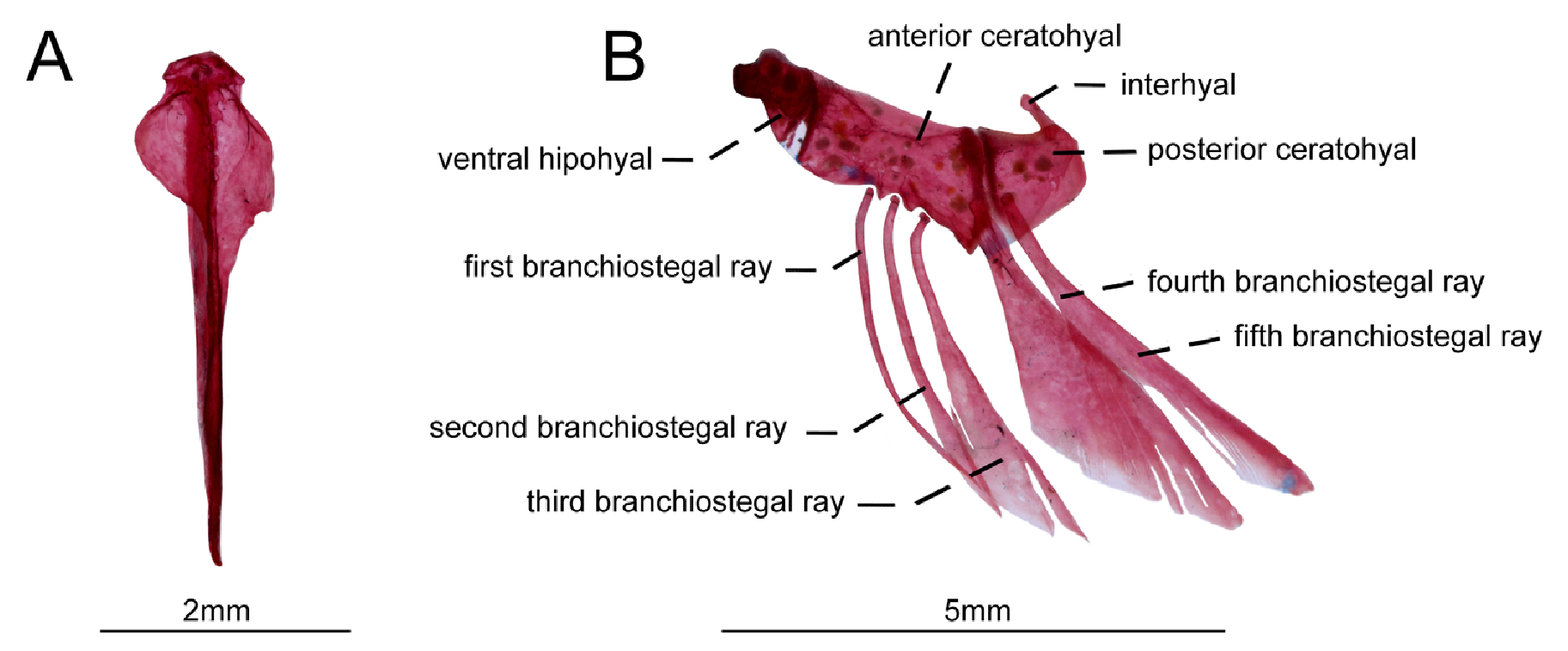
Hyoid arch. Head of urohyal is anteriorly expanded with two short anterolateral arms each articulating with hypohyal dorsal. Body of urohyal with two contralateral ridges conferring a diamond shape. Free urohyal blade somewhat as long as urohyal body. Dorsal hipohyal somewhat rounded in shape not visible in lateral view. Ventral hipohyal somewhat triangular in shape with socket that contacts urohyal. Anterior ceratohyal rectangular. Posterior ceratohyal triangular, its relative length as long as ventral hypohyal. Branchiostegal rays five (5). First and second branchiostegal thin. Third to fifth branchiostegal spatulated. First to fourth branchiostegal rays attached to anterior ceratohyal. Fifth branchiostegal ray attached to posterior ceratohyal ( Fig. 6 View FIGURE 6 ).
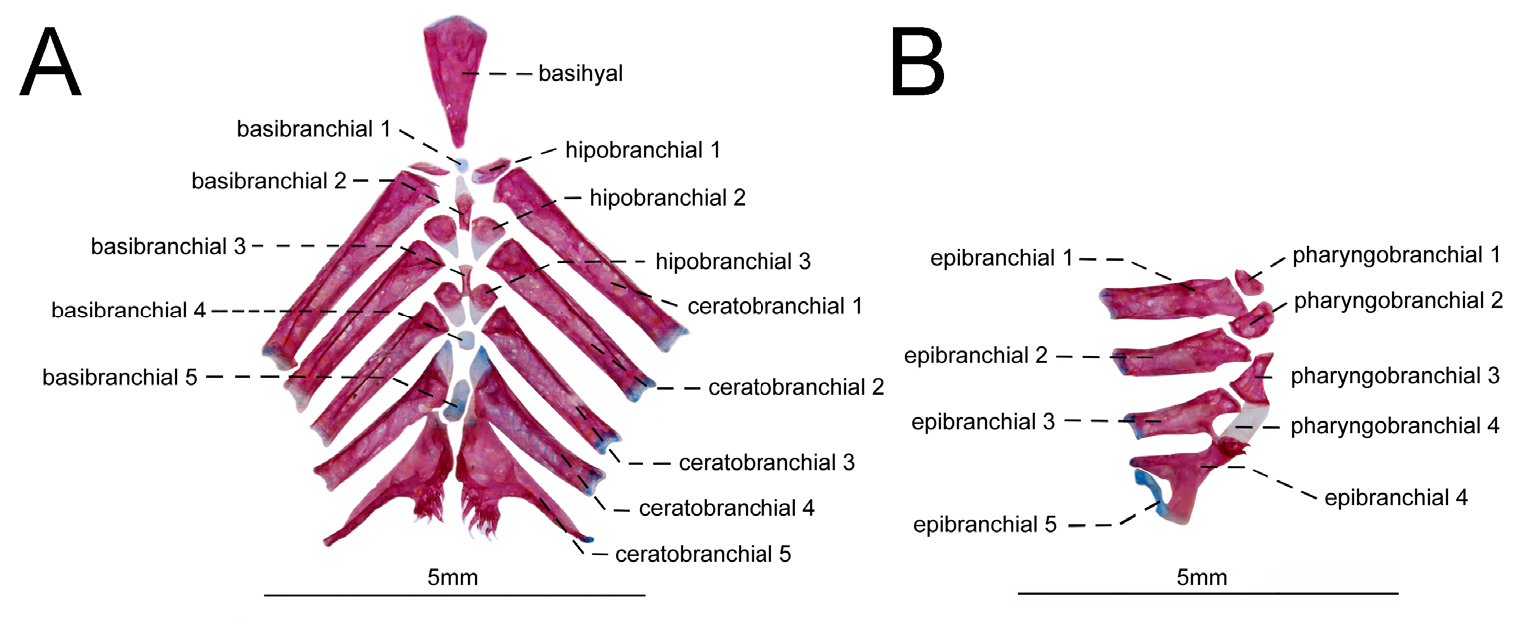
Branchial arches. All gill rakers short and unossified attached on anterior margin of each branchial arch. Pair of tiny spine shape ossicles present at base of each gill raker. Gill rakers on first branchial arch nine (1), 10(3), or 11(3). Basihyal somewhat triangular in shape, anterior portion twice as wide as its posterior portion. First basibranchial unossified, round in shape. Second and third basibranchials thin and ossified. Fourth basibranchial unossified and somewhat hexagonal in shape. Fifth basibranchial unossified and trapezoidal in shape, posterior margin twice as large as anterior margin. All hypobranchials with ossified and cartilaginous portion. First hipobranchial triangular. Second and third hipobranchials somewhat rectangular in shape. Ceratobranchials 1 to 4 retangular shape. First ceratobranchial longest, ceratobranchials 2 to 4 gradually decreasing in size. Ceratobranchial 5 supports lower pharyngeal plate, which anchors eight (1), nine (3), 10(2), or 11*(1) teeth ( Fig. 7 View FIGURE 7 ).
Epibranchials 1 to 4 ossified. Epibranchials 1 to 3 rectangular shaped. First epibranchial longest, second and third epibranchials gradually decreasing in size. Third epibranchial with posterior spine shaped process. Fourth epibranchial somewhat Y-shaped. Fifth epibranchial cartilaginous, tiny, articulating with both posterior arms of fourth epibranchials. Pharyngobranchials 1 to 3 ossified. First and second pharyngobranchial ovoid. Third pharyngobranchial triangular in shape with cartilaginous anterior portion. Fourth pharyngobranchial cartilaginous and rectangular in shape. Upper pharyngeal plate between fourth pharyngobranchial and fourth epibranchial. Upper pharyngeal plate with four (1), six (2), seven (7), or nine*(3) teeth.
Neurocranium. Mesethmoid oriented at about 45° angle from vomer, until reaching anterior margin of frontals; anterior portion with small lateral process. Frontal convex in lateral profile, about 60% as long as skull. Antorbital process of frontal present. Antorbital portion of frontal shorter than half-length of orbit. Anterior portion of anterior fontanelle limited by contralateral posterior processes of mesethmoid and completely surrounded by frontals. Posterior fontanelle about 90% as long as anterior fontanelle. Anterior one third of posterior fontanelle surrounded by frontals, posterolateral portion by parietals and posterior edge by supraoccipital. Parietal contacts frontal anteriorly, supraoccipital posteriorly, epioccipital and pterotic laterally ( Fig. 5 View FIGURE 5 ).
Lateral ethmoid a small element Y-shaped positioned in a vertical thought contact between mesethmoid and frontals; connected ventrally to parasphenoid by a connective tissue and to frontals by two strong and short ligaments. Posterodorsal process of lateral ethmoid shorter than main axis of this bone. Orbitosphenoid connected dorsally to neurocranium and posteriorly separated from pterosphenoid by a segment of cartilage. Entire ventral surface of orbitosphenoid contacting dorsal margin of parasphenoid. Pterosphenoid contacts orbitosphenoid anteriorly, and associates with frontal dorsally. Pterosphenoid contacts parasphenoid only posteroventrally, with its anteroventral surface not contacting dorsal margin of parasphenoid, forming a lateral fenestra.
Vomer arrow shaped anteriorly, with small anterior processes on each side, becoming larger posteriorly and diverging in two posterior processes, contacting anterior margin of parasphenoid. Posterior process of vomer shorter than vomer largest width. Anterior portion of vomer, which corresponds to distance from its anterior margin to the anteriormost contact with the endopterygoid, is longer than its posterior portion, which extends from the anteriormost contact with the endopterygoid until posteriormost margin of this bone. Anteriorly, parasphenoid reaches posterior portion of vomer, posteriorly surrounding ventral margin of prootics and ventral surface of basioccipital. Antorbital portion of parasphenoid shorter than its orbital portion. At its posterolateral portion, parasphenoid contacts posteroventral margin of pterosphenoid through a tapered lateral process; and dorsally contacts orbitosphenoid entirely.
Sphenotic somewhat triangular with anterior process that extends from its anterodorsal to its anteroventral margins. Sphenotic contacts pterosphenoid anteriorly, frontal anterodorsally, pterotic posterodorsally, prootic posteroventrally, and parasphenoid only at its ventralmost tip. Prootic contacts basioccipital, exoccipital, pterotic, sphenotic and pterosphenoid through cartilage filled sutures, and directly contacts to parasphenoid. Prootic with single prominent foramen. Pterotic form posterolateral portion of skull roof, and contacts prootic and exoccipital ventrally, epioccipital posteriorly, parietal dorsally, frontal anterodorsally and sphenotic anteriorly. Parietal, epioccipital, and pterotic bones in contact, posttemporal fossa absent.
Supraoccipital contacts parietal anteriorly and epioccipital posterolaterally, extending dorsally to dorsal margin of parietal. Epioccipital form posterodorsal corner of neurocranium and contacts supraoccipital medially. Exoccipital contacts basioccipital ventrally, pterotic anterodorsally, prootic anteriorly, and epioccipital posterodorsally. Internally, basioccipital and exoccipitals form a pair of chambers for cf. asteriscus otoliths; and pterotics and prootics allocate a pair of cf. lapillus otoliths located anterodorsally to basioccipital and exoccipitals chambers. Baudelot’s ligament partially ossified.
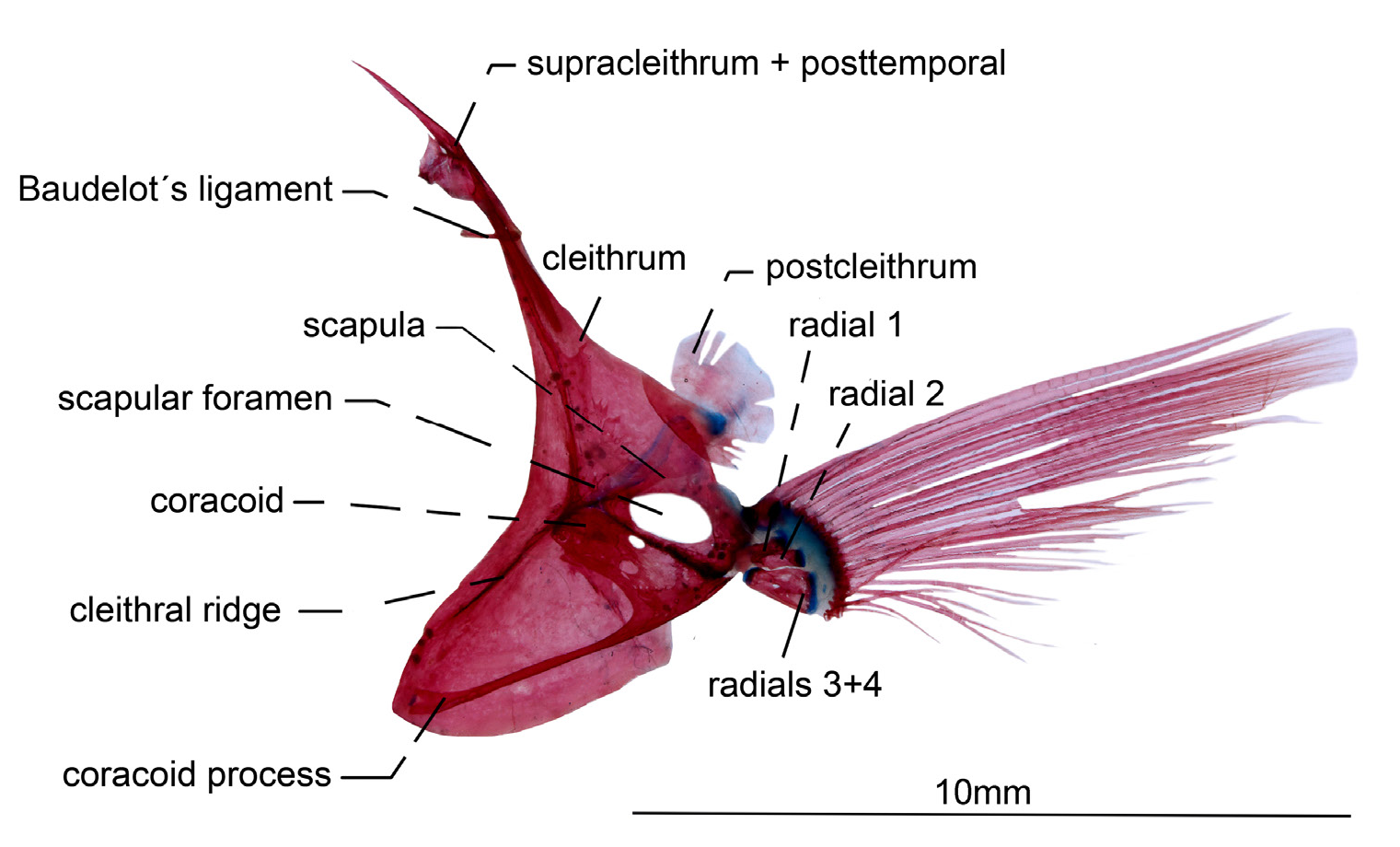
Pectoral fin and girdle. Cleithrum somewhat half-moon shaped. Cleithral ridge not limiting anterodorsal border of this bone. Posterior process of cleithrum approximately triangular. Postcleithrum thin and discoid. Supracleithrum fused with posttemporal and lying at posterolateral surface of neurocranium at epioccipitals and pterotic sutures. Baudelot’s ligament partially ossified. Coracoid with long anteroventral process that contacts cleithral ridge at anteriormost portion of cleithrum. Mesocoracoid absent. Scapula with large foramen. Radials 1 and 2 independents. Radials 3 and 4 co-ossified. Pectoral-fin tip not beyond body cavity ( Fig. 8 View FIGURE 8 ).
Weberian complex. Supraneural with long anterior process that contacts supraoccipital. Scaphium somewhat triangular in shape. Intercalarium tiny located between scaphium and tripus. Tripus elongated. Parapophysis of second and fourth vertebra in contact and ventrally curved. Neural arch of third vertebrae well developed. Neural spine of fourth vertebra long and spine shaped ( Fig. 5 View FIGURE 5 ).
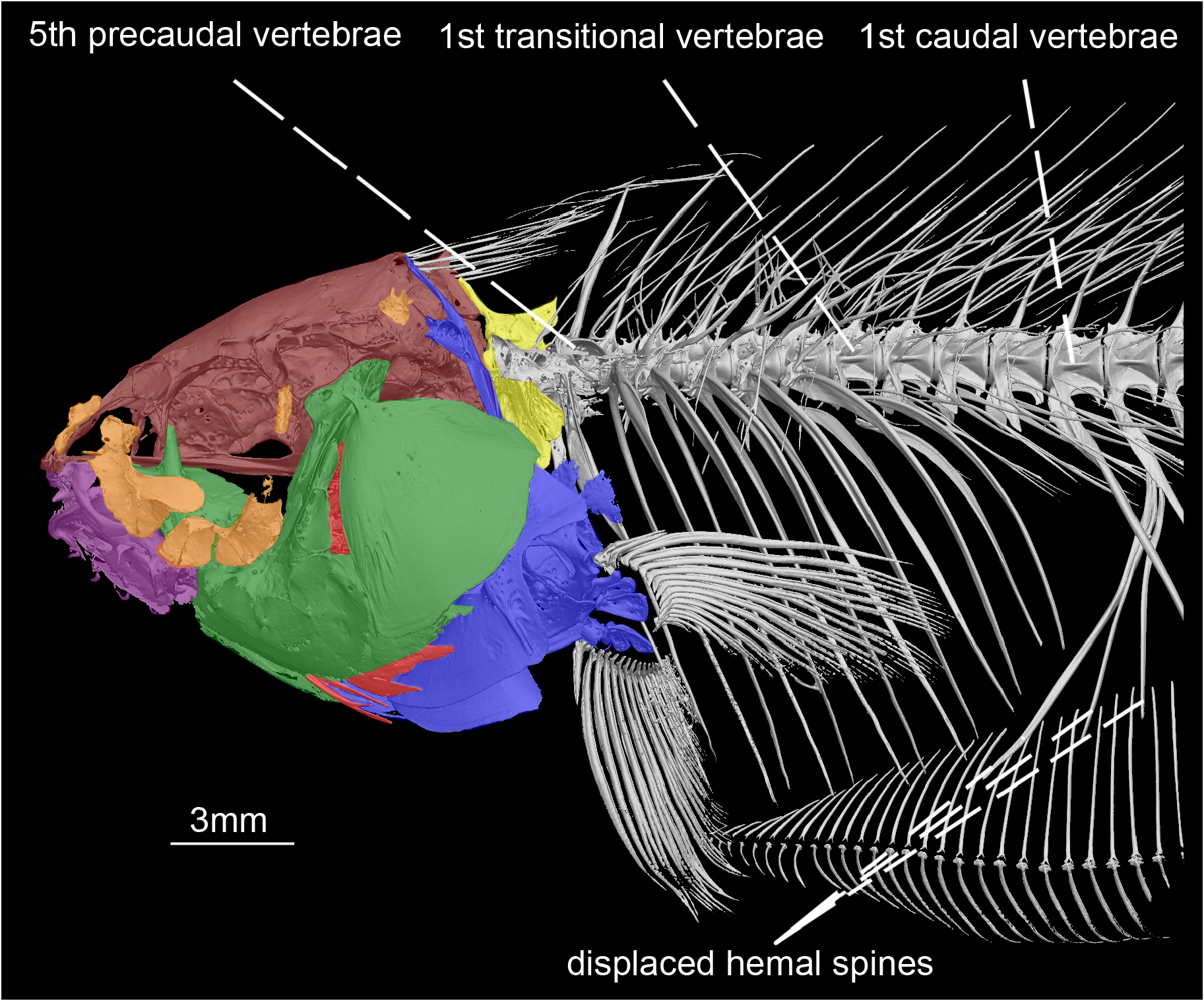
Axial skeleton. Precaudal vertebrae 13(6), or 14*(4). Transitional vertebrae three (2), four*(7), or five (1). Pleural ribs six (4), or seven*(6). Relative length of two anteriormost post-Weberian ribs approximately as long as abdominal cavity depth. Displaced hemal spines two (1), three*(6), or four (3). Relative size of hemal spine of 26 th to 30 th vertebrae longer than its associated pterygiophore ( Fig. 5 View FIGURE 5 ).
Intermuscular bones at 7–9 th vertebrae. Epineurals highly branched. Rami arranged in several directions conferring a stellate shape for these elements. Epicentrals unbranched. “Epipleurals” present ( Fig. 5 View FIGURE 5 ).
Karyotype. Diploid chromosome number 2n = 36 ( Sene et al., 2014).
Etymology. The epithet “ catira ” is in reference to a popular dance in Brazilian folklore performed by herdsmen and farmers in the areas of influence of the “sertaneja” culture in São Paulo and Mato Grosso do Sul states, which includes the occurrence area of the new species occurs. A noun in apposition.
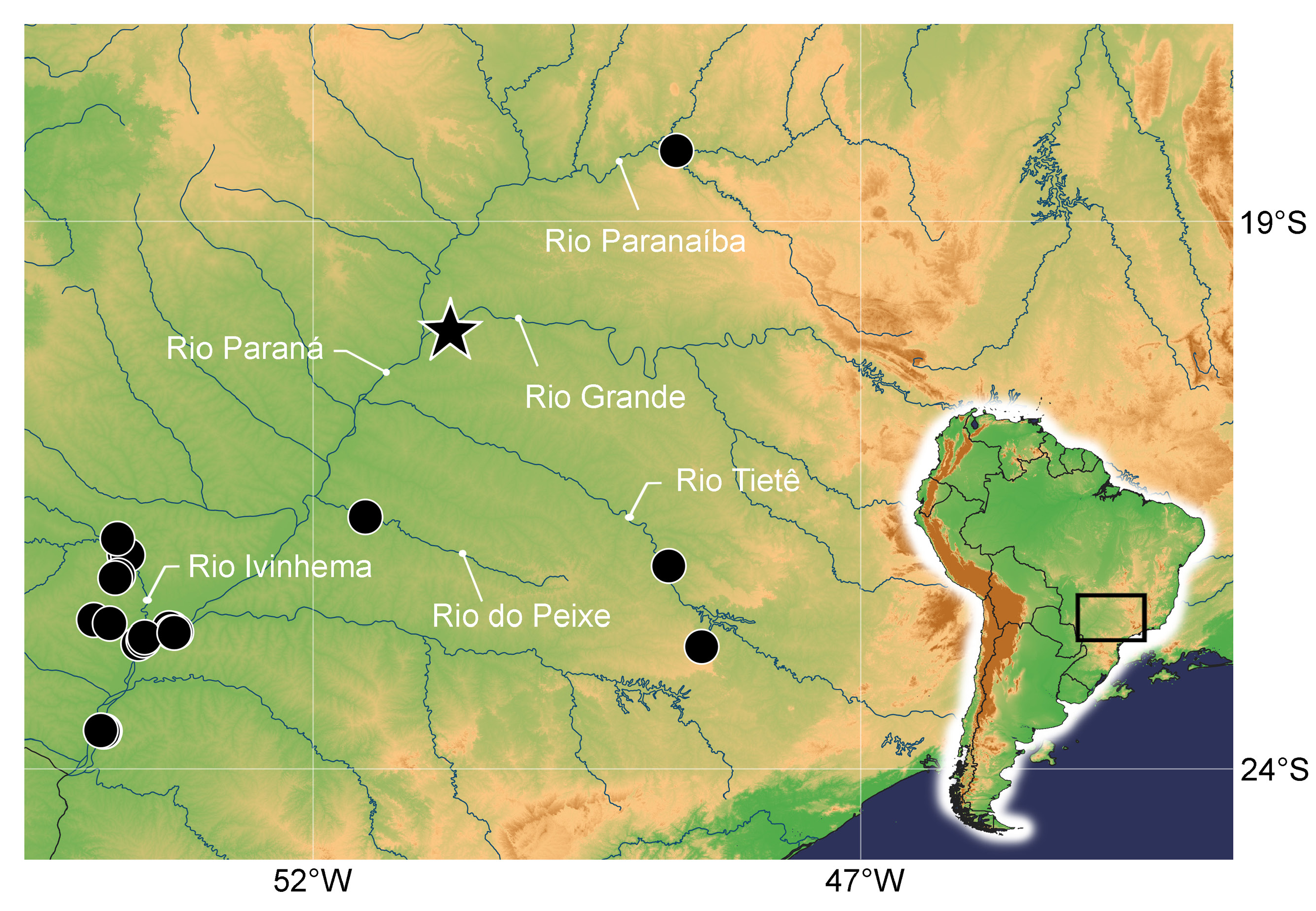
Geographical distribution. Eigenmannia catira is known from rio Grande , rio Ivinhema , rio Paraná, rio Paranaíba, rio do Peixe, and rio Tietê, upper rio Paraná basin, Brazil ( Fig. 9 View FIGURE 9 ).
Conservation status. Eigenmannia catira has a relatively broad distribution, widespread in the upper rio Paraná basin. Therefore, according to the International Union for Conservation of Nature ( IUCN) categories and criteria ( IUCN Standards and Petitions Subcommittee, 2022), E. catira can be classified as Least Concern ( LC).
TABLE 2 | Morphometry of the examined specimens of Eigenmannia catira. N = number of samples analyzed, SD = standard deviation.
| Holotype | Range | N | Mean | SD | |
|---|---|---|---|---|---|
| Total length (mm) | 164.9 | 57.8–285.8 | 27 | – | – |
| Length to end of anal fin (mm) | 132.7 | 43.8–217.2 | 31 | – | – |
| Head length (mm) | 15.8 | 7.1–25.5 | 35 | – | – |
| Caudal-filament length (mm) | 39.6 | 12.6–84.4 | 27 | – | – |
| Percents of length to end of anal fin | |||||
| Caudal-filament length | 29.8 | 13.3–41.1 | 27 | 28.5 | 7.6 |
| Body depth at pectoral fin | 15.9 | 14.9–19.4 | 31 | 17.3 | 1.2 |
| Body depth at anal fin | 14.4 | 12.2–18.4 | 30 | 15.3 | 1.5 |
| Body width | 5.1 | 4.2–7.8 | 31 | 6.0 | 0.7 |
| Pre-anal distance | 15.0 | 13.8–19.9 | 30 | 16.8 | 1.4 |
| Pre-pectoral distance | 12.9 | 12.7–16.7 | 31 | 14.4 | 0.9 |
| Anal-fin length | 80.8 | 79.8–91.0 | 30 | 84.0 | 2.7 |
| Pectoral-fin length | 8.1 | 7.2–13.5 | 31 | 9.8 | 1.4 |
| Snout to anus | 6.9 | 5.4–15.0 | 30 | 9.2 | 2.1 |
| Head length | 11.9 | 11.7–16.2 | 31 | 13.1 | 0.9 |
| Percents of head length | |||||
| Head width at opercle | 54.6 | 53.3–73.0 | 35 | 58.2 | 4.3 |
| Head width at orbits | 44.9 | 41.8–58.4 | 35 | 47.9 | 4.0 |
| Head depth at supraoccipital | 80.5 | 74.3–90.0 | 35 | 83.8 | 3.7 |
| Head depth at orbits | 64.5 | 54.6–71.9 | 35 | 62.4 | 3.9 |
| Snout length | 29.1 | 27.3–33.6 | 35 | 29.9 | 1.5 |
| Snout to posterior naris distance | 20.8 | 18.8–25.9 | 35 | 22.2 | 1.9 |
| Posterior naris to orbit distance | 8.1 | 3.5–12.0 | 35 | 7.3 | 1.8 |
| Postorbital distance | 55.3 | 50.6–64.9 | 35 | 55.3 | 3.5 |
| Opercular opening | 26.2 | 22.6–40.9 | 34 | 29.8 | 3.8 |
| Internarial width | 15.1 | 12.0–21.5 | 35 | 17.0 | 2.2 |
| Internasal distance | 9.1 | 7.5–15.7 | 35 | 10.4 | 1.7 |
| Interorbital distance | 35.0 | 29.4–38.8 | 35 | 34.5 | 2.3 |
| Orbital diameter | 20.3 | 14.5–24.5 | 35 | 19.8 | 2.3 |
| Maxilla length | 23.4 | 14.4–25.2 | 35 | 19.4 | 2.7 |
| Oral width | 19.7 | 15.5–22.7 | 35 | 19.3 | 1.8 |
| Percents of caudal-filament length | |||||
| Caudal-filament width | 1.1 | 0.7–8.2 | 21 | 1.8 | 1.8 |
| Caudal-filament depth | 7.2 | 4.1–16.5 | 21 | 7.9 | 2.7 |
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
| LEA |
University of Lethbridge |
| R |
Departamento de Geologia, Universidad de Chile |
| CS |
Musee des Dinosaures d'Esperaza (Aude) |
| V |
Royal British Columbia Museum - Herbarium |
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Order |
|
|
Family |
|
|
Genus |
Eigenmannia catira
| Cardoso, Vinicius de Carvalho & Dutra, Guilherme Moreira 2023 |
Eigenmannia trilineata
| Lopez & Castello 1966 |