Piromis arenosus Kinberg, 1867
|
publication ID |
https://doi.org/ 10.5281/zenodo.277211 |
|
DOI |
https://doi.org/10.5281/zenodo.6183581 |
|
persistent identifier |
https://treatment.plazi.org/id/D34C87B8-4D35-2627-FF44-FCEE66F9FADE |
|
treatment provided by |
Plazi |
|
scientific name |
Piromis arenosus Kinberg, 1867 |
| status |
|
Piromis arenosus Kinberg, 1867 View in CoL
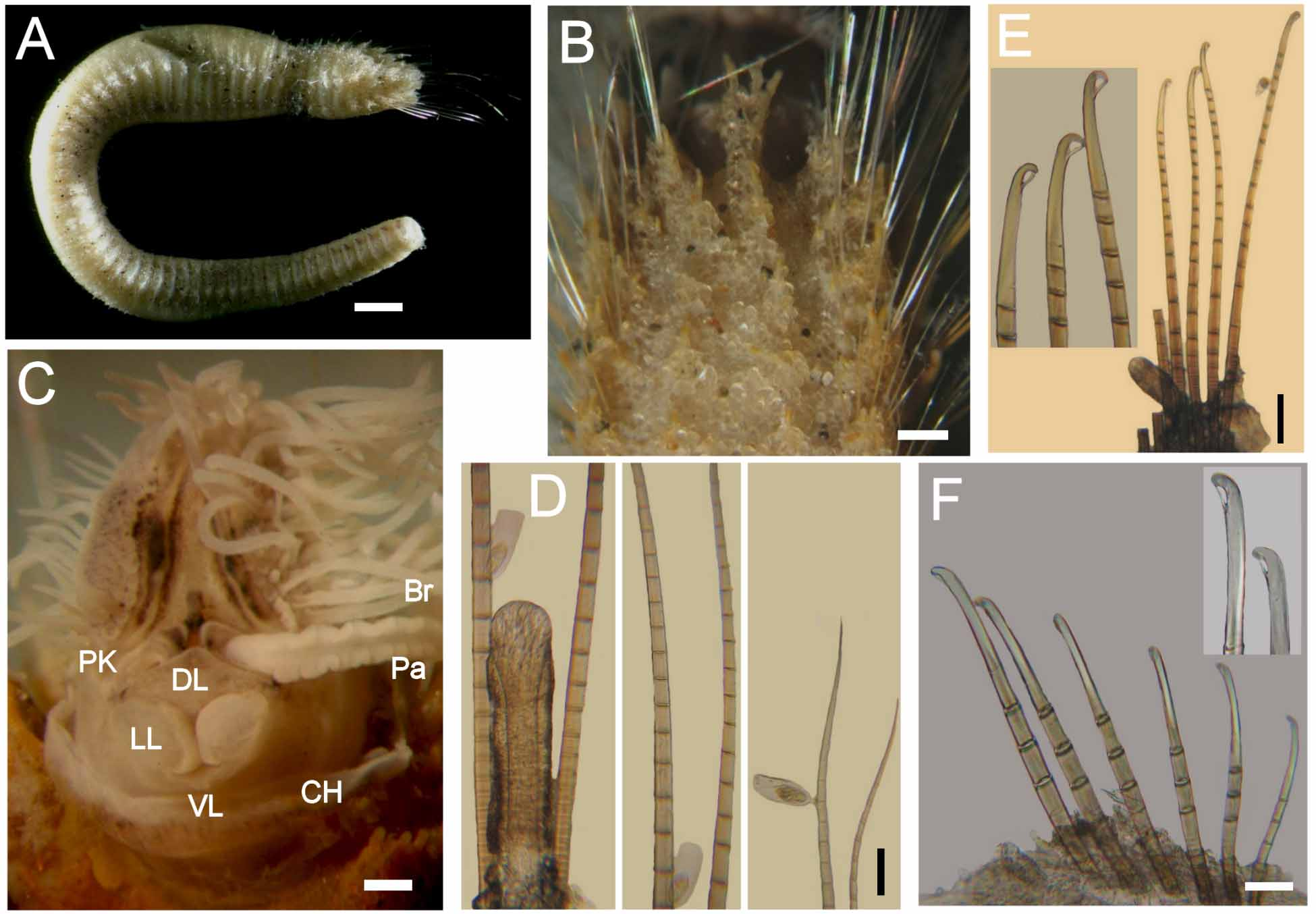
Figure 1 View FIGURE 1
Piromis arenosus Kinberg, 1867:338 View in CoL ; Kinberg, 1910:68, Pl. 26, Fig. 3 View FIGURE 3 A–G (redescr.); Hartman, 1949:117, Pl. 15, Figs. 7–9 View FIGURE 7 View FIGURE 8 View FIGURE 9 ; Hartman, 1961:122, Pl. 27, Figs. a–c; Day, 1961:509 Fig. 8 View FIGURE 8 e; Hartman, 1966:44, Pl. 1, Figs. 10–13 View FIGURE 10 View FIGURE 11 View FIGURE 12 View FIGURE 13 ; Day, 1967:664 Figs. 32.4a–d.
Trophonia capensis McIntosh, 1885:363 View in CoL –364, Pl. 44, Figs. 7–9 View FIGURE 7 View FIGURE 8 View FIGURE 9 , Pl. 33a, Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ; McIntosh, 1904:52 –53.
Type material. Southern Africa. Syntypes of Piromis arenosus (SMNH-502, another tag in the same vial NHRM- 803), Port Natal, South Africa, Wahlberg coll. Holotype of Trophonia capensis (BMNH-85.12.1.260), mature female, damaged, Sea Point, near Cape Town, South Africa, intertidally, Dec. 1873.
Additional materials. Two specimens (LACM-AHF-2506), rocky shore, The Haven Hotel, Transkei coast, Eastern Cape, South Africa, 2 Dec. 1938, J.H. Day coll. (notopodial papillae as long as 1/5 chaetal length). A mature female broken in two pieces (LACM-AHF -2507), rocky shore, Sea Point, Cape Town, KwaZulu-Natal, South Africa, 12 Feb. 1938, J.H. Day coll. A thin specimen (LACM-AHF-2508) without data, University of Cape Town Ecological Survey, Sta. CH 3J, 8 Jan. 1940.
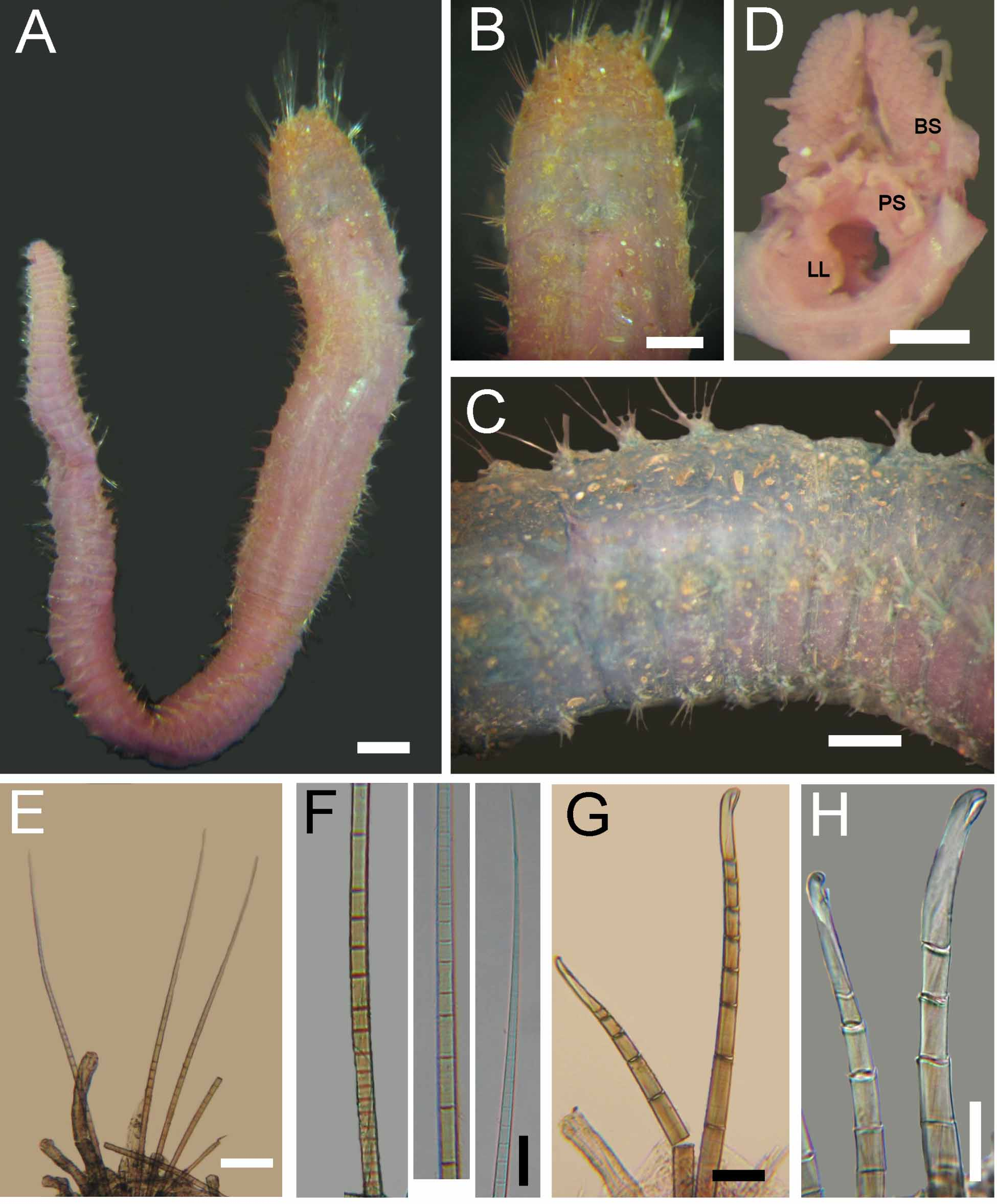
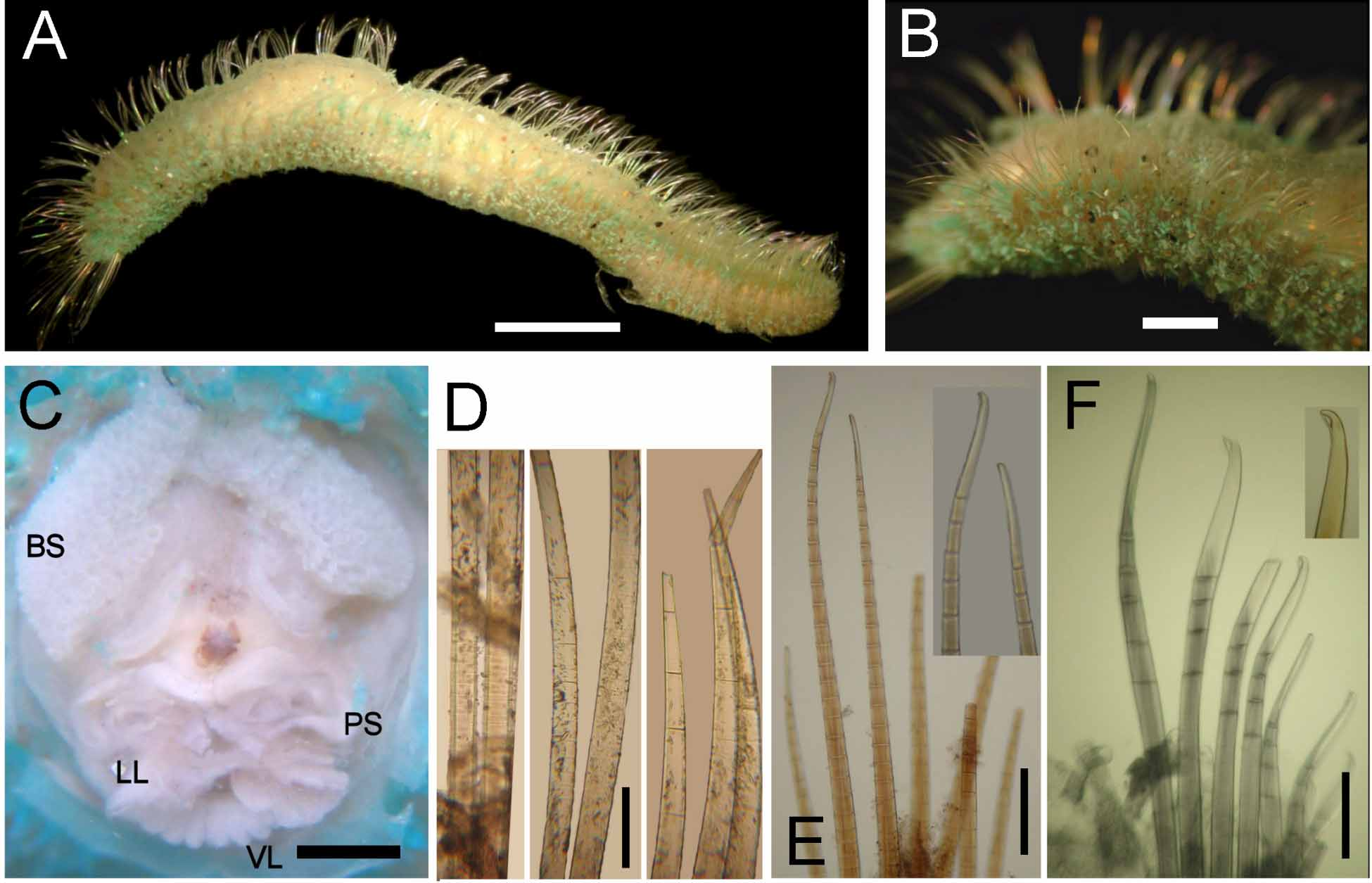
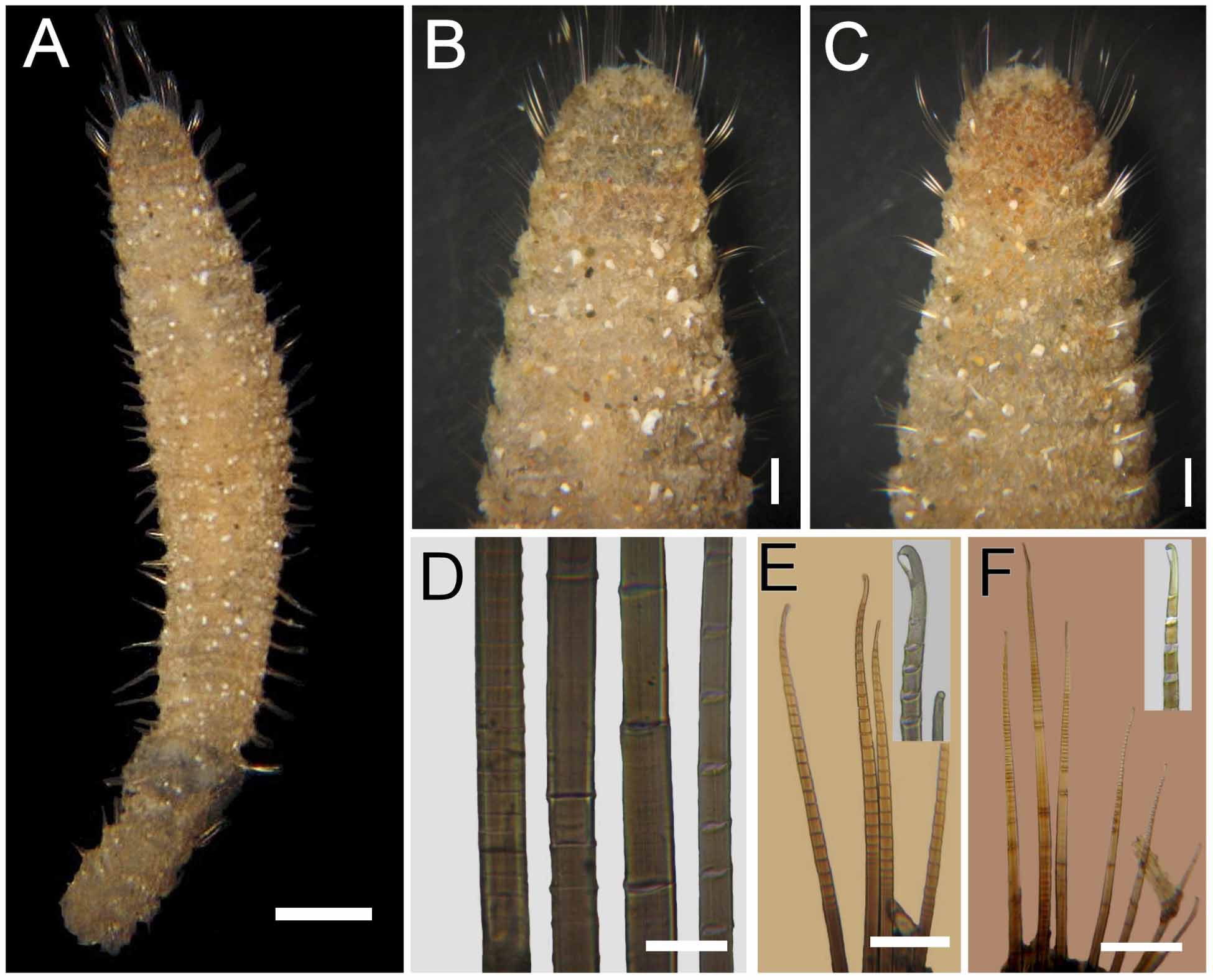
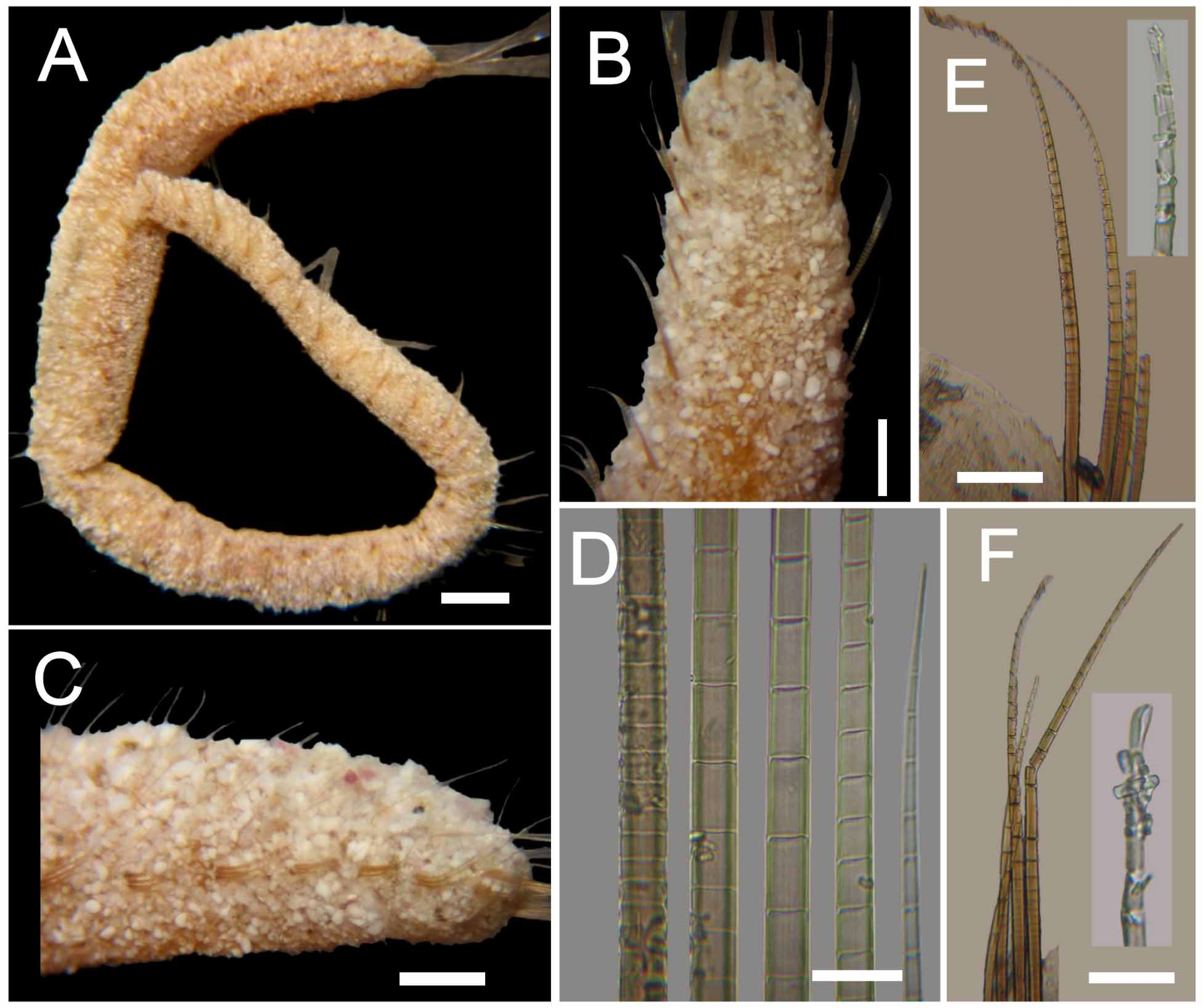
Description. Syntypes apparently fixed in alcohol, damaged, smaller one almost broken into two pieces (employed for redescription, body with more consistency), larger syntype softer, in three pieces ( Fig. 1 View FIGURE 1 A). Body clavate, slightly tapering posteriorly, regenerating in anterior and posterior ends; tunic papillated, with small sediment particles mostly embedded in tunic ( Fig. 1 View FIGURE 1 B, C). Papillae arranged in longitudinal rows, barely exposed dorsally, four papillae per segment, more exposed ventrally (six papillae per segment). Complete syntype 54 mm long, 6.5 mm wide, cephalic cage 6 mm long, 75 chaetigers.
Cephalic hood not exposed in syntypes (not dissected to avoid further damage); short, margin smooth in smaller specimen. Anterior end not studied in syntypes; tongue-shaped branchial lobe exposed, branchiae lost. A non-type specimen (LACM-AHF -5210) already dissected; prostomium elevated cone with median black longitudinal band; two large red eyes present (dark brown in holotype). Caruncle well developed, running to tip of branchial plate; median keel pale, lateral ridges black ( Fig. 1 View FIGURE 1 D). Palps long, thick; palp keels rounded, elevated, dark (pale in holotype). Lateral lips well-developed; ventral and dorsal lips well-developed, punctuated with black spots (pale, eroded in holotype). Branchiae cirriform, arising from tongue-like plate, arranged in two lateral groups, each group with branchiae arranged in about 26 oblique rows, basal rows with more branchiae, each group with about 100 filaments. Nephridial lobes not seen.
Cephalic cage chaetae mostly damaged, 1/9 as long as whole body, or slightly shorter than body width. Chaetigers 1–3 involved in the cephalic cage; chaetae arranged in short rows, dorsolateral in chaetiger 1, lateral in chaetigers 2–3; 6–8 chaetae per fascicle. Anterior dorsal margin of chaetiger 1 with a large middorsal black scar. Anterior chaetigers with short papillae, mostly eroded, about 1/7 as long as notochaetae; chaetigers 2–8 with elevated smooth lobe formed by long postchaetal papilla. Chaetigers 1–3 of about same length (difficult to determine, because of regeneration in larger syntype). Chaetal transition from cephalic cage to body chaetae abrupt; compound bidentate neurohooks from chaetiger 6. Gonopodial lobes not seen.
Parapodia well developed, forming large dorsal lobes in chaetigers 1–8. Parapodia lateral, median neuropodia ventrolateral. Noto- and neuropodia with long chaetal lobes and short digitate papillae; both with three small prechaetal and three larger postchaetal papillae (seen in smaller specimen).
Median notochaetae arranged in transverse row, 6–8 per bundle, as long as 1/2 –1/3 body width; all notochaetae multiarticulated capillaries with short articles basally and medially; slightly longer distally, with hooked tips ( Fig. 1 View FIGURE 1 E). Neurochaetae multiarticulated capillaries in chaetigers 1–5; multiarticulated bidentate hooks from chaetiger 6, arranged in transverse row ( Fig. 1 View FIGURE 1 F), hooks in posterior chaetigers arranged in J-shaped pattern, with six chaetae in anterior and median chaetigers, up to 7–9 posterior chaetigers ( Fig. 1 View FIGURE 1 G). Anterior neurohooks slightly tapered, gently curved distally; posterior neurohooks with distal article medially swollen, more markedly curved distally, accessory tooth often eroded.
Posterior end conical; pygidium with anus terminal, without cirri ( Fig. 1 View FIGURE 1 C).
Variation. The holotype of Trophonia capensis has most chaetae broken, most papillae eroded, and the anterior end exposed; it was previously dissected middorsally along almost entire length of body. Holotype 60 mm long, 4 mm wide, cephalic cage 3 mm long, 81 chaetigers; oocytes 120 µm.
Remarks. Piromis arenosus Kinberg, 1867 includes Trophonia capensis McIntosh, 1885 , both described from the same region in Southern Africa. Kinberg (1910), and Hartman (1949) illustrated the typical hooked tip with a smaller accessory tooth, but Hartman (1961) illustrated an almost straight hook with a hooded tip, with the hood almost completely covering the fang. Those figures may be based on a different species. Most neurohooks in syntypes and additional specimens have lost the accessory tooth or hood, although some show a low hump. The only exception is a small, 3.5 mm wide specimen (LACM-AHF), that has the anterior end exposed, and has most neurohooks with the accessory tooth still in place. Perhaps the lack of an accessory tooth in most specimens would explain why Hartman identified the specimens as Semiodera capensis , but this stems from confusion about Semiodera . The species belonging in this genus lack a thick tunic, have a cephalic shield and short anchylosed neurohooks; those species having a thick tunic, lacking a dorsal shield and anchylosed neurohooks should be transferred to Trophoniella Caullery, 1944 .
On the other hand, the middorsal trifid lobe is confirmed in the smaller specimen; it has a basal thickening that carries three marginal lobes, all directed anteriorly on the same plane. The marginal lobes are bifid, with the junction area darker. This complete structure, the trifid lobe, feature is fragile and often breaks off from the specimens leaving a scar. The dorsal notopodial lobes are more developed on chaetigers 1–6. Further, the size-related features are the notopodial lobes, which become more prominent in more posterior chaetigers in larger specimens, and the number of neurohooks.
Monro (1933) and Day (1955) both regarded S. kinsemboanus Augener, 1918 as a junior synonym of Piromis arenosus Kinberg, 1867 . However, some specimens from Western Africa have the same long notopodial papillae and neurochaetae that Augener described from his only specimen. These are relevant differences and therefore they are regarded as separate species (see below).
One of the intertidal South African specimens (LACM-AHF-5229) has very large sediment particles and a very different appearance from the type materials. The neurohooks are arranged in a J-shaped pattern from chaetiger 11, while they are in such a pattern from chaetiger 9 in the typical P. arenosus specimens. It is included here with hesitation pending some better preserved specimens.
Piromis arenosus View in CoL is closely allied with P. kisemobanus ( Augener, 1918) n. comb., n. spell., a tropical Western African species, since both species have sediment grains mostly embedded in the tunic. They differ because P. arenosus View in CoL has relatively shorter notopodial papillae (about 1/4–1/5 as long as notochaetae) while they are longer in P. kisemboanus (about 1/2–1/3 as long as notochaetae), and the distal article in neurohooks is medially widened and bidentate in P. arenosus View in CoL , while they are cylindrical, tapering, mostly unidentate in P. kisemboanus .
Distribution. Originally described from Natal, Southern Africa, the species may be restricted to temperate waters around Southern Africa.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Piromis arenosus Kinberg, 1867
| Salazar-Vallejo, Sergio I. 2011 |
Trophonia capensis
| McIntosh 1904: 52 |
| McIntosh 1885: 363 |
Piromis arenosus
| Day 1967: 664 |
| Hartman 1966: 44 |
| Hartman 1961: 122 |
| Day 1961: 509 |
| Hartman 1949: 117 |
| Kinberg 1910: 68 |
| Kinberg 1867: 338 |