Isoperla baumanni Szczytko & Stewart, 1984Isoperla bifurcata Szczytko & Stewart
|
publication ID |
https://doi.org/10.5281/zenodo.4760320 |
|
DOI |
https://doi.org/10.5281/zenodo.4764788 |
|
persistent identifier |
https://treatment.plazi.org/id/D27B87A3-FF9F-F100-FC2C-FD5A9621FE79 |
|
treatment provided by |
Felipe |
|
scientific name |
Isoperla baumanni Szczytko & Stewart Isoperla bifurcata Szczytko & Stewart |
| status |
|
Isoperla baumanni Szczytko & Stewart
NEW LARVA DESCRIPTION
( Figs. 1 View Fig a-d, 2 View Figs c, 5 View Figs a-h, 20 View Figs c)
Isoperla baumanni Szczytko & Stewart 1984 , 77:258-260. ♂, ♀, Ovum unknown.
Isoperla baumanni Szczytko & Stewart 2002 , 128:2-3. Larva (not reared) = I. bifurcata .
Material examined. TYPES: I. baumanni , Holotype ♂, Allotype ♀, CA: Plumas Co., Domingo Springs Campground, 6 mi (9.7 km) NW Chester, Domingo Spring, 25/VI/1980, R. Baumann, J. Stanger ( NMNH #104070). Additional Specimens. CALIFORNIA: Plumas Co., Domingo Spring, Domingo Springs Campground, 8.5 mi (13.7 km) NW Chester on Old Red Bluff Rd., 20/I/2007, J. Sandberg, Larvae; 04/II/2007, Larvae; 24/III/2007, Larvae; 09/VI/2007, J. Sandberg, A. Richards, Larvae (reared); 13/VI/2007, J. Sandberg, ♂ ♀, Larvae (reared); 17/VI/2007, ♂ ♀, Larvae (reared); 07/VII/2007, ♂ ♀ Larvae; 03/XI/2007, Larvae; 02/V/2008, J. Sandberg, D. Pickard, Larvae; 17/V/2008, J. Sandberg, Larvae; 13/VI/2008, Larvae (reared); 20/VI/2008, ♂ ♀ Larvae (reared); 22/VI/2008, ♂ ♀, Larvae; 05/VI/2010, Larvae (reared); 03/VII/2010, ♂ ♀.
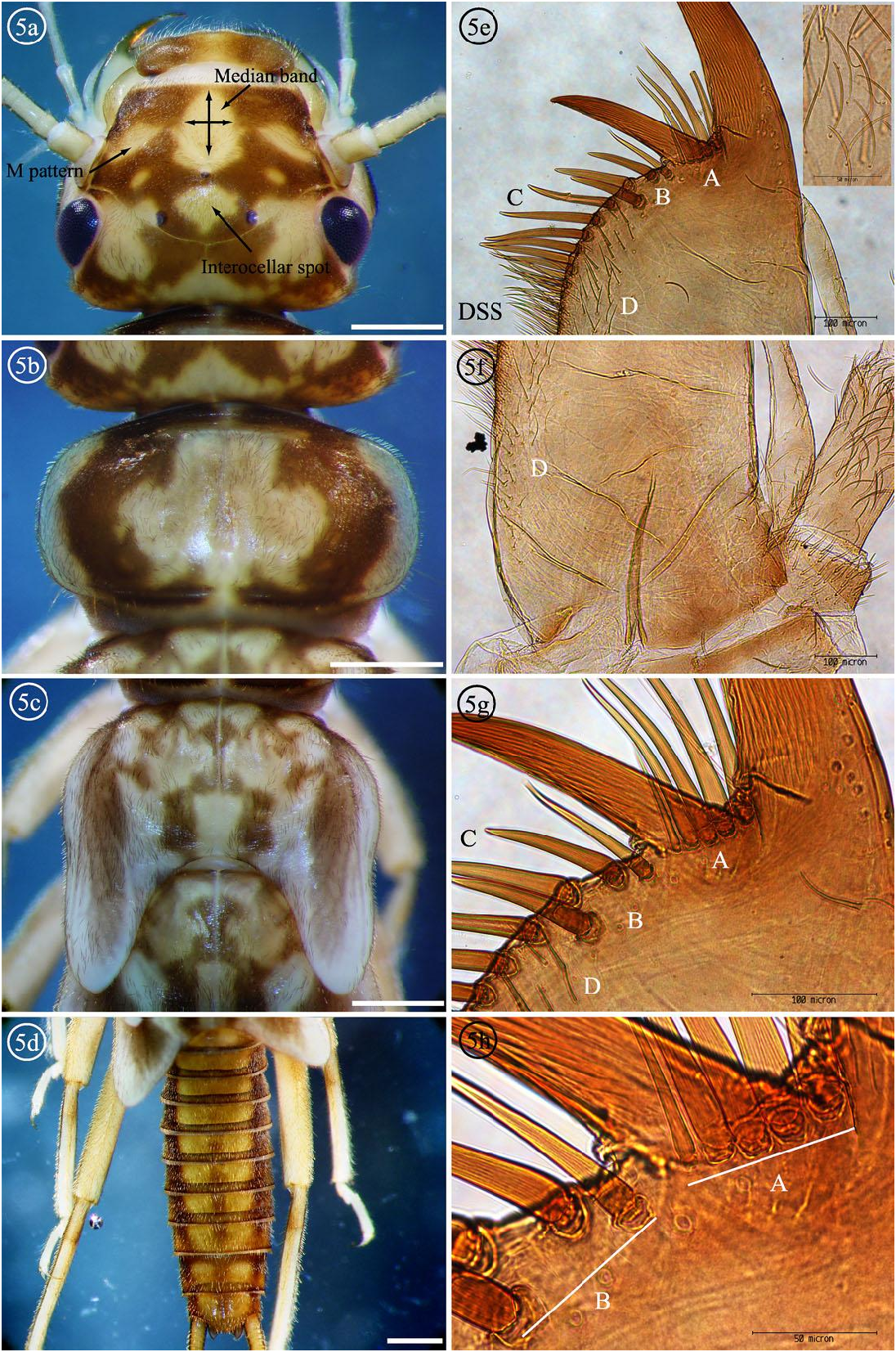
Male larva. Body length of mature larva 9–10 mm. Dorsum of head with contrasting pigment pattern and fine dark clothing setae, anterior frontoclypeus margin unpigmented; light M shaped pattern anterior to median ocellus usually connected to light frontoclypeus area by an apically narrowed median longitudinal light band, its width at mid length approximately equal to the width at base, lateral arms with irregular margins usually disconnected from median light band, directed posterolaterally, and extending to antennal bases; posterior ocelli with partially enclosed large light areas along outer lateral margins; interocellar area partially light, completely enclosed by dark pigment, light area extending past posterior ocelli, not reaching dark pigment below the arms of the epicranial suture, light area generally oval shaped with acutely constricted base; occiput with irregular spinulae band extending from below eye to near median epicranial suture, completely enclosed by dark pigment ( Figs. 1d View Fig , 5a View Figs ). Lacinia bidentate, total length 1069–1256 µm ( Figs. 1 View Fig , 2c View Figs , 5e- h View Figs , Tables 2-4 View Table 2 View Table 3 View Table 4 ); submarginal row (A+B) with 7–10 setae, groups A-B interrupted by gap below subapical tooth (SAT) inner margin ( Fig. 5g View Figs ); 5–7 submarginal setae (A) in a close set row beginning at base of apical tooth (AT), ending before reaching SAT inner margin, row usually single, rarely 2 setae thick, plus 1 thin marginal setae ( TMS) adjacent to AT inner margin, sometimes obstructed from view by the AT, submarginal setae (A), or broken, and 1 dorsal seta (DS) located below SAT inner margin, sometimes obstructed from view by SAT, submarginal setae (B), or broken ( Fig. 5h View Figs ); 2–4 submarginal setae (B) located past SAT inner margin ( Figs. 5 View Figs g-h); 15–27 marginal setae (C) initially longstout and widely spaced, usually several setae near end of row arranged in pairs protruding at dorsal and ventral angles, last few shorter and closer, blending into and difficult to differentiate from dorsal surface setae ( Fig. 5e View Figs ); 38–71 ventral surface setae (D) scattered below submarginal and marginal setae, ending posteriorly at approximately ¾ the inner lacinia margin length, occasionally a few setae below submarginal row striated ( Fig. 5f View Figs ); dorsal surface setae ( DSS) forming dense, laterally protruding, longitudinal band, concentrated at junction with marginal setae (C), ending at approximately ½ or a little more the lacinia length ( Fig. 5e View Figs ). Galea with 15–33 setae in sparse ventral row, apex with 2–3 setae. Maxillary Palp segments 2–3 with curved apically rounded setae ( Fig. 5e View Figs Inset). Pronotum with broad median light area bordered by thick dark comma shaped bands typical of the I. sobria complex; discs each with thick black clothing setae, those along median margins usually enclosed by indistinct light brown pigment, lateral margins with broad light bands ( Fig. 5b View Figs ). Meso and metanotum with contrasting pigment pattern and fine dark clothing setae ( Fig. 5c View Figs ). Legs with numerous fine golden clothing setae and scattered erect spines on outer surface of femora, erect spines longest and concentrated along dorsal surfaces; fine silky setae sparse on dorsal surface of femora, numerous and continuous on tibia ( Fig. 20c View Figs ); tibia with faint transverse bands near proximal end. Abdominal terga with three longitudinal dark stripes; wide light median longitudinal band bisected with thin light brown median longitudinal stripe present on distal segments; lateral pair of dark longitudinal stripes about twice as wide as median dark stripe, extending to lateral margins; numerous fine dark clothing setae and erect spines scattered dorsally; posterior margin with scattered long and numerous short spines in a concentrated row ( Fig. 5d View Figs ).
Distribution. Northern California, known only from one Sierra Nevada high elevation spring.
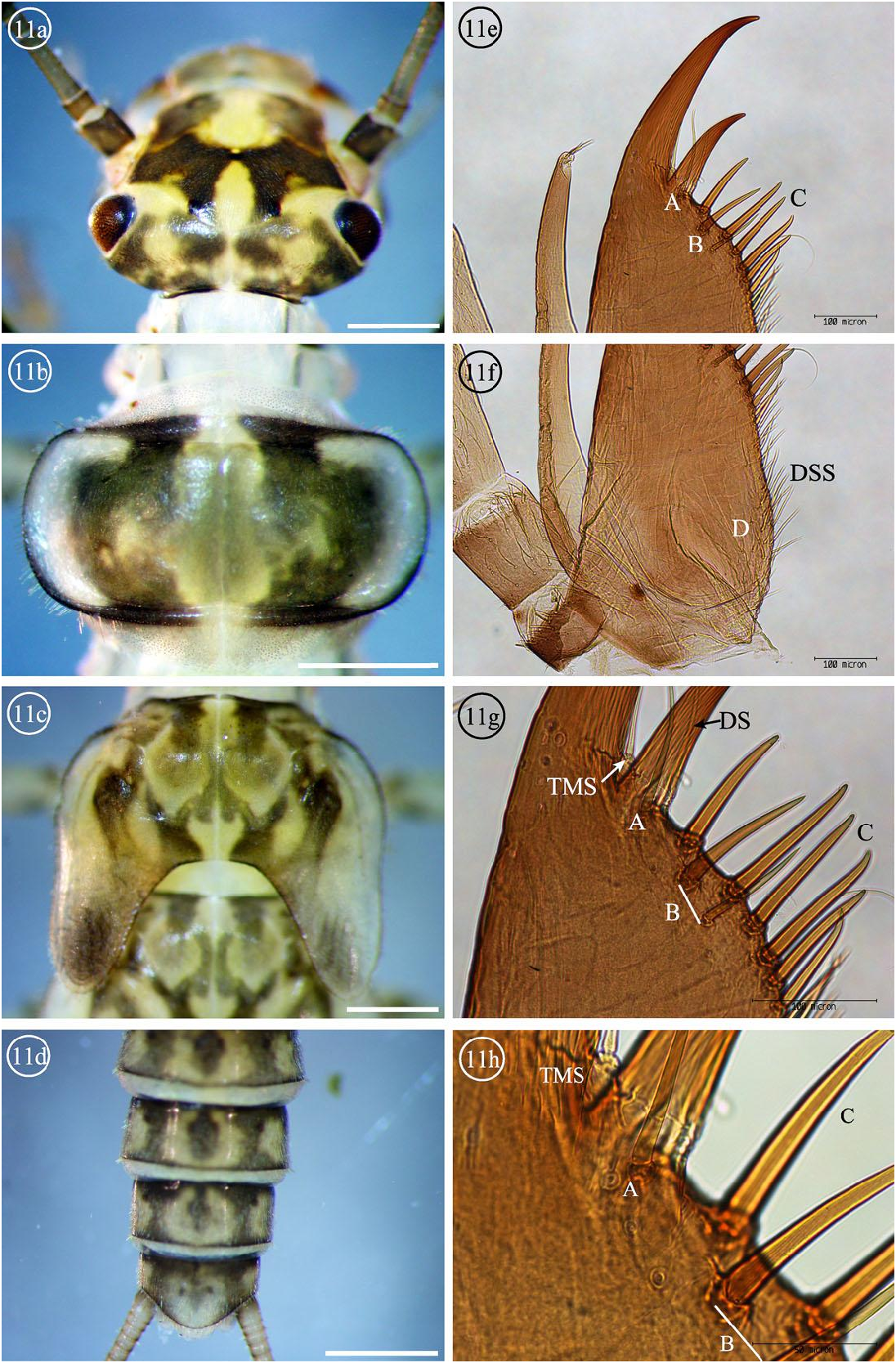
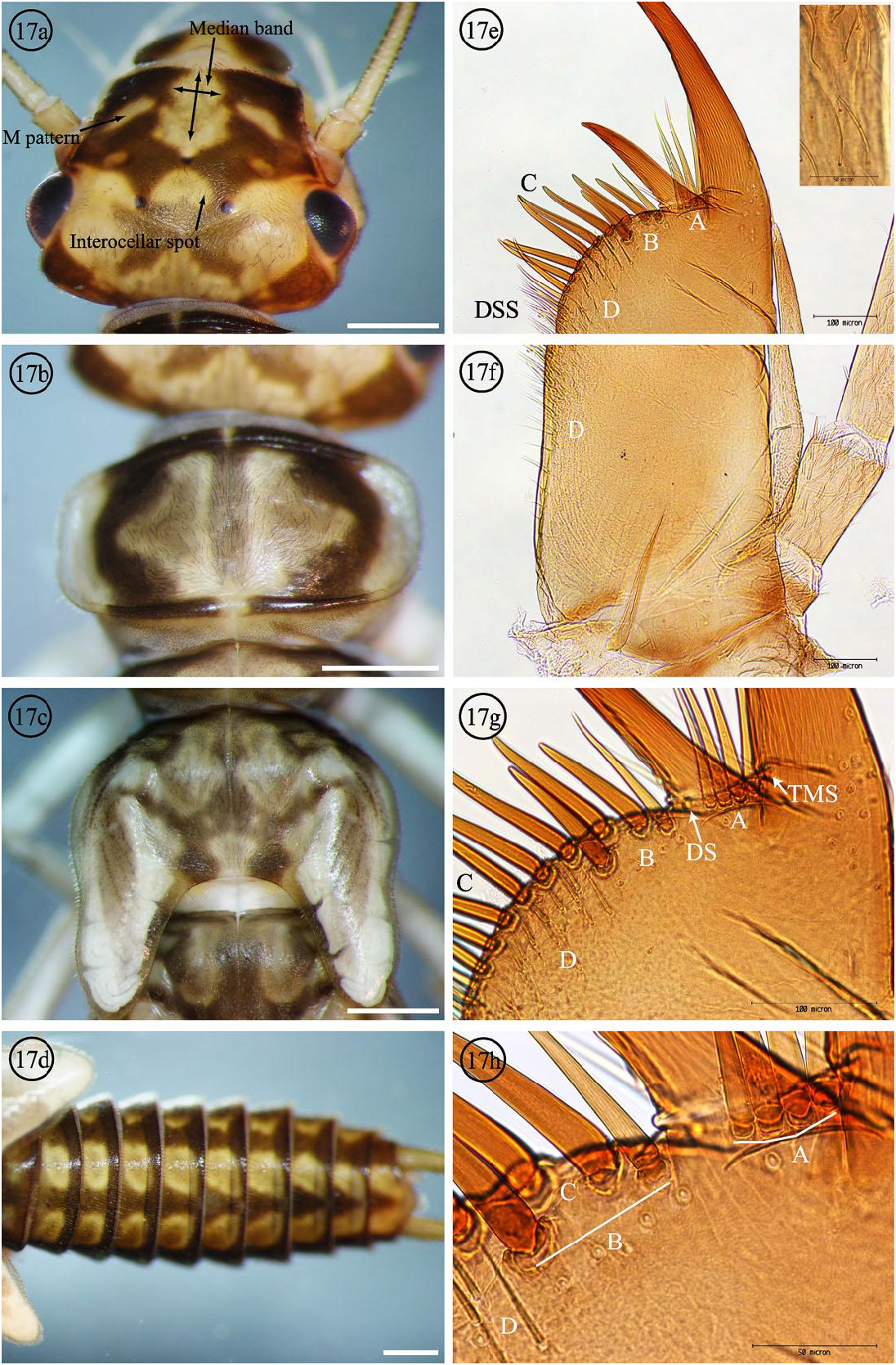
Diagnosis. The 5–7 submarginal (A) setae of I. baumanni male larvae ( Figs. 5 View Figs g-h) are most similar to Isoperla sobria (Hagen) and I. tilasqua but differs from I. miwok with only one submarginal (A) seta ( Fig. 11g View Figs ). The setae counts alone were not sufficient to separate the three species with multiple close set submarginal (A) setae; I. sobria with 3–4 submarginal (A) setae ( Figs. 17e View Figs , g-h) and 2-5 submarginal (A) setae for I. tilasqua ( Figs. 19e View Figs , g-h). It is suspected that increased variation will be observed as more populations are studied. The basal segments 2–3 of the maxillary palpi have long, thin, apically rounded setae ( Fig. 5e View Figs Inset) which are shared by I. tilasqua ( Fig. 19e View Figs Inset); I. sobria has long thin, apically pointed setae ( Fig. 17e View Figs Inset). The variably shaped and large I. baumanni interocellar light area is completely enclosed by dark pigment ( Fig. 5a View Figs ), similar to I. sobria ( Fig. 17a View Figs ), but the latter is usually an inverted V shape (or sometimes a very small light spot). The interocellar light area of I. tilasqua (Meacham Creek, Oregon population) is not completely enclosed by dark pigment, and continues past the posterolateral ocelli to the median base of the dorsal head capsule ( Fig. 19a View Figs ). The light M shaped dorsal head pattern of I. tilasqua is a continuous line, but is interrupted in I. baumanni and I. sobria . A median longitudinal light band is present in all three species, but this line is approximately half as wide as the median base of the light M pattern in I. tilasqua ( Fig. 19a View Figs ).
Remarks. Isoperla baumanni may be restricted to Domingo Spring, Plumas County, a high volume and nearly stenothermic spring and emergence occurred in June–July. It was absent in nearby smaller Mosquito Spring containing both I. bifurcata and I. sobria , and an unnamed spring tributary to Gurnsey Creek with only I. bifurcata present. Recent collecting and rearing at Domingo Springs from 2006–2010 confirm three sympatric species: I. baumanni , I. bifurcata , and I. marmorata (three collected adults only). Isoperla bifurcata larvae were distributed throughout the spring and collected from all available submerged substrates (aquatic moss, aquatic plants, wood and rock). Isoperla baumanni larvae were primarily collected from submerged aquatic liverworts located in a small area along the southeastern bank, below the footbridge and above the cattle fence.
Everted I. baumanni males (reared & collected) did not compare well with the original description and appears to be the result of using two methods for everting the aedeagus. The aedeagus illustrated by Szczytko & Stewart (1984 – Fig. 53) was cleared and then hyper everted and this method may not be directly comparable to males that were everted live. Attempts to produce a similar evertion as illustrated in Szczytko & Stewart (1984) resulted in bursting the aedeagus membrane. In the current study, 31 reared and fully everted males lacked the projected, conical, anterodorsal striated, bladelike tip. Instead, a small depression was visible in this area and when the freshly everted aedeagus was fixed in near boiling water, seminal fluid streamed from the depression. The absence of long, paired membranous lobes was also observed in I. fulva , I. miwok , and I. roguensis , emphasizing the difference between live everted specimens of the current study and hyper everted preserved specimens illustrated in previous studies. Perhaps the striated bladelike tip of the I. baumanni aedeagus as illustrated in Szczytko & Stewart (1984) is an internal valve, which controls the release of sperm during copulation.
Two mature male larvae from Long Canyon Creek, El Dorado County, California were prepared for maxillae examination and tentatively identified as I. baumanni . The partly mature larvae with developing wing pads were collected on April 27 th, 2007, by Austin Brady Richards near Bendorf Spring. Both the males and females in the sample possessed an inverted V shaped light mark within the interocellar area, similar to I. sobria . One unsuccessful return trip by the author was conducted in early July, 2011, to attempt rearing and adult collection.
Isoperla bifurcata Szczytko & Stewart
( Figs. 2d View Figs , 6 View Figs a-h, 20 View Figs d)
Isoperla bifurcata Szczytko & Stewart 1979 , 32:80-84, 86.
Isoperla bifurcata: Bottorff et al. 1990 , 92:299-302. Larva (reared).
Isoperla baumanni Szczytko & Stewart 2002 , 128:2-3. Larva (not reared) = I. bifurcata .
Material examined. TYPES: I. bifurcata , Holotype ♂, OR: Union Co., ~ 6 mi (9.7 km) E Medical Springs, DFTM Proj., Lick Creek, 23/VII/1975, D. Dunster ( NMNH #76343 ) ; Allotype ♀, same location and collector, 30/ VI /1976 ( NMNH); Paratype ♂ ♀, same location and collector as Holotype, 23/VII/1975 ( NMNH). Additional Specimens. CALIFORNIA: Butte Co., Butte Creek, Butte Meadows Campground, Humbug Rd., Butte Meadows, 30/III/2007, J. Sandberg, Larvae; Butte Creek, Cherry Hill Campground, Humbug Rd., 9 mi (14.5 km) NE Lomo (Hwy 32), 18/II/2007, J. Sandberg, D. Pickard, Larvae. El Dorado Co., NF Cosumnes River, Headwaters below Singleton Springs, E Grizzly Flat, 22/ VI /1987, R. Bottorff, Larvae ( NMNH); NF Cosumnes River, Headwaters below Singleton Springs, E Grizzly Flat, 22/ VI /1987, R. Bottorff, Larvae ( CAS). Fresno Co., Huntington Lake, 07/VII/1919, F. Blasdell, ♂ ( NMNH). Plumas Co., Domingo Spring, Domingo Springs Campground, 8.5 mi (13.7 km) NW Chester on Old Red Bluff Rd., 12/VIII/2006, J. Sandberg, J. Slusark, Larvae; 01/X/2006, J. Sandberg, ♂ ♀, Larvae (mature and early instar); 10/XII/2006, Larvae; 20/I/2007, Larvae; 04/II/2007, Larvae; 24/III/2007, Larvae; 22/IV/2007, Larvae (reared); 06/ V /2007, J. Sandberg, D. Pickard, Larvae; 19/ V /2007, J. Sandberg, S. Hassur, Larvae (reared); 28/ V /2007, J. Sandberg, D. Pickard, Larvae (reared); 09/ VI /2007, J. Sandberg, A. Richards, ♂, Larvae (reared), Exuviae; 13/ VI /2007, J. Sandberg, ♂ ♀, Larvae (reared); 17/ VI /2007, ♂ ♀; 07/VII/2007, ♂ ♀; 01/VIII/2007, ♀, Larvae; 16/IX/2007, ♂ ♀, Larvae; 21/IX/2007, ♂ ♀, Larvae; 30/IX/2007, ♀; 03/XI/2007, ♂ ♀, Larvae; 20/ V /2008, J. Sandberg, D. Pickard, Larvae; 17/ V /2008, Larvae; 13/ VI /2008, ♂ ♀; 20& 22/ VI /2008, ♂ ♀; 06/IX/2008, ♂ - ♀; 05/ VI /2010, Larvae (reared); 03/VII/2010, ♂; Mosquito Creek (East Branch), 0.6 mi (1 km) E of Domingo Springs, 7.9 mi (12.7 km) W of Chester, 04/II/2007, J. Sandberg, Larvae; 01/IV/2007, Larvae; Mosquito Creek (West Branch), 0.4 mi (0.6 km) E of Domingo Springs, 8.1 mi (13.0 km) W of Chester, 04/II/2007, J. Sandberg, Larvae; 24/III/2007, Larvae; 22/IV/2007, J. Sandberg, D. Pickard, Larvae (reared); 19/ V /2007, J. Sandberg, S. Hassur, Larvae (reared); 28/ V /2007, J. Sandberg, D. Pickard, ♂, Larvae (reared); 13/ VI /2007, J. Sandberg, ♂ ♀, Larvae (reared); 01/VIII/2007, Larvae; 11/VIII/2007, Larvae; 16/IX/2007, Larvae; 21/IX/2007, Larvae; 03/XI/2007, Larvae; 17/ V /2008, Larvae; 13/ VI /2008, Larvae (reared); 20/ VI /2008, ♂ ♀, Larvae (reared); 05/ VI /2010, Larvae; 03/VII/2010, ♂ ♀. Siskiyou Co., Big Springs Creek, Big Springs Park, Nixon Rd., Mt. Shasta, 13/ V /2007, J. Sandberg, D. Pickard, Larvae. Tehama Co., Spring Trib of Gurnsey Creek, Gurnsey Creek Campground, Hwy 36, 2.3 mi (3.7 km) N Hwy 32 Intersec., 26/IV/2010, R. Baumann, B. Kondratieff, A. Richards, J. Sandberg, J. Slusark, Larvae (reared). Trinity Co.,?, Carrville,?/ VI /1913, E.C. VanDyke, ♂ ♀ ( CAS). Tulare Co., Marble Fork Kaweah River, Sequoia Nat. Pk., 24/VII/1907, J. Bradley, ♂ ( NMNH). OREGON: Union Co., Lick Creek, about 6 mi (9.6 km) E Medical Springs, 30/ VI /1976, D. Dunster, ♀ ( NMNH).
Male larva. Body length of mature larva 9–11 mm. Dorsum of head with contrasting pigment pattern and fine dark clothing setae, anterior frontoclypeus margin unpigmented; light M shaped pattern anterior to median ocellus connected to light frontoclypeus area by broad median longitudinal light band, lateral thin arms directed posterolaterally, and extending to antennal bases; posterior ocelli with completely enclosed small light areas along outer lateral margins; interocellar area partially light and variable, from completely enclosed by dark pigment to open posteriorly and connected to light area below occipital spinulae band; occiput with irregular spinulae band extending from below eye to near median epicranial suture, enclosed by dark and light brown pigment ( Fig. 6a View Figs ). Lacinia bidentate, total length 666–967 µm ( Figs. 2d View Figs , 6e- h View Figs , Tables 2-4 View Table 2 View Table 3 View Table 4 ); submarginal row (A+B) with 2–4 setae, groups A-B interrupted by gap below subapical tooth (SAT) inner margin ( Figs. 6 View Figs g-h); 1–2 submarginal setae (A) the first inserted at base of apical tooth (AT) inner margin, the second when present, located between SAT and AT inner margins, plus 1 thin marginal seta ( TMS) adjacent to AT inner margin, sometimes obstructed from view by AT, submarginal setae (A), or broken, and 1 dorsal seta (DS) located below SAT inner margin, partially obstructed by SAT or submarginal setae (B) ( Figs. 6 View Figs g-h); 1–2 submarginal setae (B) located past SAT inner margin ( Figs. 6 View Figs g-h); 8–11 marginal setae (C) initially long-stout and widely spaced, last few shorter and closer, blending into and difficult to differentiate from dorsal surface setae ( Fig. 6e View Figs ); 17–38 ventral surface setae (D) scattered below submarginal and marginal setae ending posteriorly at approximately ¾ the inner lacinia margin length ( Fig. 6f View Figs ); dorsal surface setae ( DSS) forming dense, laterally protruding, longitudinal band on and along inner-lateral margin, ending before posterior-most ventral surface seta ( Fig. 6f View Figs ). Galea with 37–50 setae in thick ventral band, apex with 4–6 setae. Maxillary Palp segments 2–3 with curved, apically pointed setae. Pronotum generally light brown, median light area bordered laterally by dark brown pigment and light rugosites; discs each with thin, dark brown comma shaped areas near lateral margins and fine dark clothing setae, lateral margins with broad light bands ( Fig. 6b View Figs ). Meso and metanotum with contrasting pigment pattern and fine dark clothing setae ( Fig. 6c View Figs ). Legs with numerous fine dark clothing setae and scattered erect spines on outer surface of femora, erect spines longest and concentrated along dorsal surfaces; fine silky setae sparse on dorsal surface of femora, numerous but not continuous on tibia ( Fig. 20d View Figs ); tibia with very faint transverse bands near proximal end. Abdominal terga with two distinct longitudinal dark stripes; wide light median longitudinal band occasionally appears bisected by muscle attachment scars; lateral pair of dark longitudinal stripes usually not extending to lateral margins; numerous fine dark clothing setae and erect spines scattered dorsally; posterior margin with scattered long and numerous short spines in a concentrated row ( Fig. 6d View Figs ).
Distribution. Northern California (Sierra Nevada high elevation springs and creeks), Idaho, and Oregon.
Diagnosis. Male larvae of I. bifurcata are most similar to I. acula and can be separated by having fine silky setae sparse on dorsal surface of femora, numerous but not continuous on tibia ( Fig. 20d View Figs ), and abdomen without a distinct median dark stripe ( Fig. 6d View Figs ).
Remarks. This species was the lone Isoperla species or occurred with either I. baumanni or I. sobria in small to large, mid-elevation spring creeks in the Sierra Nevada range of California. Emergence was extended at Domingo and Mosquito Springs, Plumas County, and occurred in May–October. An extended summer-fall emergence was also observed for I. laucki .
Isoperla bifurcata Szczytko & Stewart
( Figs. 2d View Figs , 6 View Figs a-h, 20 View Figs d)
Isoperla bifurcata Szczytko & Stewart 1979 , 32:80-84, 86.
Isoperla bifurcata: Bottorff et al. 1990 , 92:299-302. Larva (reared).
Isoperla baumanni Szczytko & Stewart 2002 , 128:2-3. Larva (not reared) = I. bifurcata .
Material examined. TYPES: I. bifurcata , Holotype ♂, OR: Union Co., ~ 6 mi (9.7 km) E Medical Springs, DFTM Proj., Lick Creek, 23/VII/1975, D. Dunster ( NMNH #76343 ) ; Allotype ♀, same location and collector, 30/ VI /1976 ( NMNH); Paratype ♂ ♀, same location and collector as Holotype, 23/VII/1975 ( NMNH). Additional Specimens. CALIFORNIA: Butte Co., Butte Creek, Butte Meadows Campground, Humbug Rd., Butte Meadows, 30/III/2007, J. Sandberg, Larvae; Butte Creek, Cherry Hill Campground, Humbug Rd., 9 mi (14.5 km) NE Lomo (Hwy 32), 18/II/2007, J. Sandberg, D. Pickard, Larvae. El Dorado Co., NF Cosumnes River, Headwaters below Singleton Springs, E Grizzly Flat, 22/ VI /1987, R. Bottorff, Larvae ( NMNH); NF Cosumnes River, Headwaters below Singleton Springs, E Grizzly Flat, 22/ VI /1987, R. Bottorff, Larvae ( CAS). Fresno Co., Huntington Lake, 07/VII/1919, F. Blasdell, ♂ ( NMNH). Plumas Co., Domingo Spring, Domingo Springs Campground, 8.5 mi (13.7 km) NW Chester on Old Red Bluff Rd., 12/VIII/2006, J. Sandberg, J. Slusark, Larvae; 01/X/2006, J. Sandberg, ♂ ♀, Larvae (mature and early instar); 10/XII/2006, Larvae; 20/I/2007, Larvae; 04/II/2007, Larvae; 24/III/2007, Larvae; 22/IV/2007, Larvae (reared); 06/ V /2007, J. Sandberg, D. Pickard, Larvae; 19/ V /2007, J. Sandberg, S. Hassur, Larvae (reared); 28/ V /2007, J. Sandberg, D. Pickard, Larvae (reared); 09/ VI /2007, J. Sandberg, A. Richards, ♂, Larvae (reared), Exuviae; 13/ VI /2007, J. Sandberg, ♂ ♀, Larvae (reared); 17/ VI /2007, ♂ ♀; 07/VII/2007, ♂ ♀; 01/VIII/2007, ♀, Larvae; 16/IX/2007, ♂ ♀, Larvae; 21/IX/2007, ♂ ♀, Larvae; 30/IX/2007, ♀; 03/XI/2007, ♂ ♀, Larvae; 20/ V /2008, J. Sandberg, D. Pickard, Larvae; 17/ V /2008, Larvae; 13/ VI /2008, ♂ ♀; 20& 22/ VI /2008, ♂ ♀; 06/IX/2008, ♂ - ♀; 05/ VI /2010, Larvae (reared); 03/VII/2010, ♂; Mosquito Creek (East Branch), 0.6 mi (1 km) E of Domingo Springs, 7.9 mi (12.7 km) W of Chester, 04/II/2007, J. Sandberg, Larvae; 01/IV/2007, Larvae; Mosquito Creek (West Branch), 0.4 mi (0.6 km) E of Domingo Springs, 8.1 mi (13.0 km) W of Chester, 04/II/2007, J. Sandberg, Larvae; 24/III/2007, Larvae; 22/IV/2007, J. Sandberg, D. Pickard, Larvae (reared); 19/ V /2007, J. Sandberg, S. Hassur, Larvae (reared); 28/ V /2007, J. Sandberg, D. Pickard, ♂, Larvae (reared); 13/ VI /2007, J. Sandberg, ♂ ♀, Larvae (reared); 01/VIII/2007, Larvae; 11/VIII/2007, Larvae; 16/IX/2007, Larvae; 21/IX/2007, Larvae; 03/XI/2007, Larvae; 17/ V /2008, Larvae; 13/ VI /2008, Larvae (reared); 20/ VI /2008, ♂ ♀, Larvae (reared); 05/ VI /2010, Larvae; 03/VII/2010, ♂ ♀. Siskiyou Co., Big Springs Creek, Big Springs Park, Nixon Rd., Mt. Shasta, 13/ V /2007, J. Sandberg, D. Pickard, Larvae. Tehama Co., Spring Trib of Gurnsey Creek, Gurnsey Creek Campground, Hwy 36, 2.3 mi (3.7 km) N Hwy 32 Intersec., 26/IV/2010, R. Baumann, B. Kondratieff, A. Richards, J. Sandberg, J. Slusark, Larvae (reared). Trinity Co.,?, Carrville,?/ VI /1913, E.C. VanDyke, ♂ ♀ ( CAS). Tulare Co., Marble Fork Kaweah River, Sequoia Nat. Pk., 24/VII/1907, J. Bradley, ♂ ( NMNH). OREGON: Union Co., Lick Creek, about 6 mi (9.6 km) E Medical Springs, 30/ VI /1976, D. Dunster, ♀ ( NMNH).
Male larva. Body length of mature larva 9–11 mm. Dorsum of head with contrasting pigment pattern and fine dark clothing setae, anterior frontoclypeus margin unpigmented; light M shaped pattern anterior to median ocellus connected to light frontoclypeus area by broad median longitudinal light band, lateral thin arms directed posterolaterally, and extending to antennal bases; posterior ocelli with completely enclosed small light areas along outer lateral margins; interocellar area partially light and variable, from completely enclosed by dark pigment to open posteriorly and connected to light area below occipital spinulae band; occiput with irregular spinulae band extending from below eye to near median epicranial suture, enclosed by dark and light brown pigment ( Fig. 6a View Figs ). Lacinia bidentate, total length 666–967 µm ( Figs. 2d View Figs , 6e- h View Figs , Tables 2-4 View Table 2 View Table 3 View Table 4 ); submarginal row (A+B) with 2–4 setae, groups A-B interrupted by gap below subapical tooth (SAT) inner margin ( Figs. 6 View Figs g-h); 1–2 submarginal setae (A) the first inserted at base of apical tooth (AT) inner margin, the second when present, located between SAT and AT inner margins, plus 1 thin marginal seta ( TMS) adjacent to AT inner margin, sometimes obstructed from view by AT, submarginal setae (A), or broken, and 1 dorsal seta (DS) located below SAT inner margin, partially obstructed by SAT or submarginal setae (B) ( Figs. 6 View Figs g-h); 1–2 submarginal setae (B) located past SAT inner margin ( Figs. 6 View Figs g-h); 8–11 marginal setae (C) initially long-stout and widely spaced, last few shorter and closer, blending into and difficult to differentiate from dorsal surface setae ( Fig. 6e View Figs ); 17–38 ventral surface setae (D) scattered below submarginal and marginal setae ending posteriorly at approximately ¾ the inner lacinia margin length ( Fig. 6f View Figs ); dorsal surface setae ( DSS) forming dense, laterally protruding, longitudinal band on and along inner-lateral margin, ending before posterior-most ventral surface seta ( Fig. 6f View Figs ). Galea with 37–50 setae in thick ventral band, apex with 4–6 setae. Maxillary Palp segments 2–3 with curved, apically pointed setae. Pronotum generally light brown, median light area bordered laterally by dark brown pigment and light rugosites; discs each with thin, dark brown comma shaped areas near lateral margins and fine dark clothing setae, lateral margins with broad light bands ( Fig. 6b View Figs ). Meso and metanotum with contrasting pigment pattern and fine dark clothing setae ( Fig. 6c View Figs ). Legs with numerous fine dark clothing setae and scattered erect spines on outer surface of femora, erect spines longest and concentrated along dorsal surfaces; fine silky setae sparse on dorsal surface of femora, numerous but not continuous on tibia ( Fig. 20d View Figs ); tibia with very faint transverse bands near proximal end. Abdominal terga with two distinct longitudinal dark stripes; wide light median longitudinal band occasionally appears bisected by muscle attachment scars; lateral pair of dark longitudinal stripes usually not extending to lateral margins; numerous fine dark clothing setae and erect spines scattered dorsally; posterior margin with scattered long and numerous short spines in a concentrated row ( Fig. 6d View Figs ).
Distribution. Northern California (Sierra Nevada high elevation springs and creeks), Idaho, and Oregon.
Diagnosis. Male larvae of I. bifurcata are most similar to I. acula and can be separated by having fine silky setae sparse on dorsal surface of femora, numerous but not continuous on tibia ( Fig. 20d View Figs ), and abdomen without a distinct median dark stripe ( Fig. 6d View Figs ).
Remarks. This species was the lone Isoperla species or occurred with either I. baumanni or I. sobria in small to large, mid-elevation spring creeks in the Sierra Nevada range of California. Emergence was extended at Domingo and Mosquito Springs, Plumas County, and occurred in May–October. An extended summer-fall emergence was also observed for I. laucki .
Bottorff, R. L., S. W. Szczytko, & A. W. Knight. 1990. Descriptions of a new species and three incompletely known species of western Nearctic Isoperla (Plecoptera: Perlodidae). Proceedings of the Entomological Society of Washington, 92 (2): 286 - 303.
Szczytko, S. W. & K. W. Stewart. 1979. The genus Isoperla (Plecoptera) of western North America; Holomorphology and systematics, and a new
Szczytko, S. W. & K. W. Stewart. 1984. Descriptions of Calliperla Banks, Rickera Jewett, and two new western Nearctic Isoperla species (Plecoptera: Perlodidae). Annals of the Entomological Society of America, 77 (3): 251 - 263.
Szczytko, S. W. & K. W. Stewart. 2002. New larval descriptions of 5 western Nearctic Isoperla: I. roguensis, I. katmaiensis and I. baumanni and further descriptions of the male and ova of I. decolorata (Plecoptera: Isoperlinae). Transactions of the American Entomological Society, 128 (1): 1 - 22.
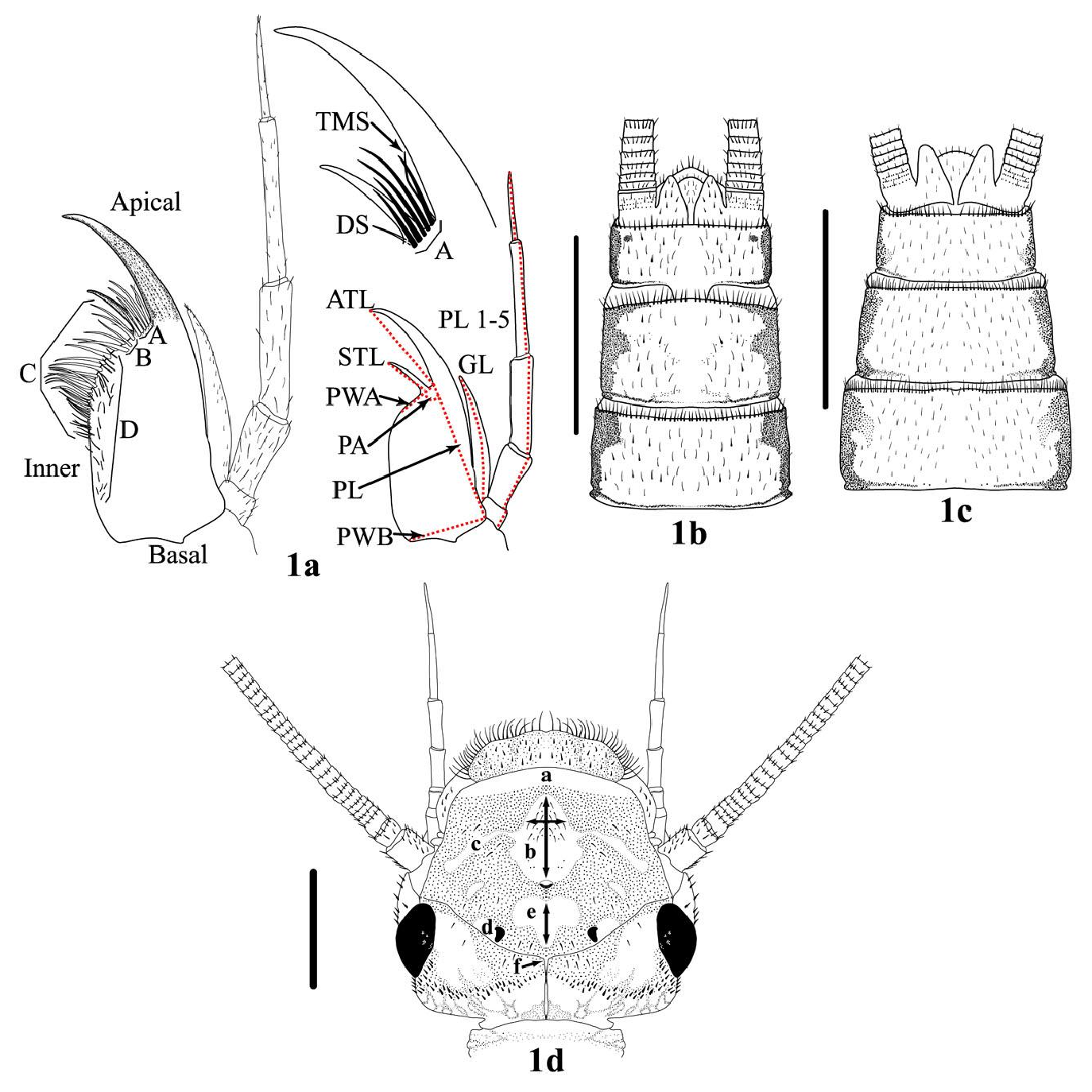
Fig. 1. Isoperla baumanni left ventral maxilla, abdominal sternites, and head, Domingo Spring, Plumas County, CA. 1a. Submarginal row groups (A) and (B), Marginal row (C), Ventral surface setae (D), Dorsal surface setae (DSS) not illustrated, Thin marginal seta (TMS), and Dorsal seta (DS) not included in submarginal row counts (Table 1). Maxilla measurements (red lines): Apical tooth length (ATL), Subapical tooth length (STL), Apical palm width (PWA), Palm angle (PA), Palm length (PL), Basal palm width (PWB), Galea length (GL), and Palp segment lengths (PL 1–5). 1b. Male and 1c. Female sterna. 1d. Dorsal head capsule: Anterior frontoclypeus area (a), Light M shaped pattern (b) and median longitudinal band (2 arrows), Lateral arms (c), Outer posterior ocellus light area (d), interocellar area (e) and length (arrow), and Epicranial suture (f). Bars = 1 mm.
Figs. 2a-q. Isoperla lacinia habitus, 40x, Bar = 500µm. 2a. I. acula; 2b. I. adunca; 2c. I. baumanni; 2d. I. bifurcata; 2e. I. denningi; 2f. I. fulva; 2g. I. laucki; 2h. I. marmorata; 2i. I. miwok; 2j. I. mormona; 2k. I. muir; 2L. I. pinta; 2m. I. quinquepunctata; 2n. I. roguensis; 2o. I. sobria; 2p. I. sordida; 2q. I. tilasqua.
Figs. 5a-h. NEW DESCRIPTION of Isoperla baumanni nymph and ventral maxilla, Domingo Spring, Plumas County, CA. Submarginal setae groups A and B, Marginal setae C, Ventral surface setae D, and Dorsal surface setae DSS. Fig. 5e Inset: Maxillary palp segment 2 with apically rounded long thin setae, 400x. White bars = 1 mm, 5e-f 100x, 5g 200x, and 5h 400x.
Figs. 20a-q. Isoperla front right leg habitus, Larvae, 32–40x, Bar = 1mm. 20a. I. acula; 20b. I. adunca; 20c. I. baumanni; 20d. I. bifurcata; 20e. I. denningi; 20f. I. fulva; 20g. I. laucki; 20h. I. marmorata; 20i. I. miwok; 20j. I. mormona; 20k. I. muir; 20L. I. pinta; 20m. I. quinquepunctata; 20n. I. roguensis; 20o. I. sobria; 20p. I. sordida; 20q. I. tilasqua.
Figs. 11a-h. Isoperla miwok nymph and ventral maxilla, Tributary of Campbell Creek, Butte County, CA. Submarginal setae groups A and B, Marginal setae C, Ventral surface setae D, Dorsal surface setae DSS, Dorsal seta DS, Thin marginal seta TMS. White bars = 1 mm, 11e-f 100x, 11g 200x, and 11h 400x.
Figs. 17a-h. Isoperla sobria nymph Fall River, Deschutes County, OR and ventral maxilla, Mosquito Spring Creek, Plumas County, CA. Submarginal setae groups A and B, Marginal setae C, Ventral surface setae D, Dorsal surface setae, Dorsal seta DS, and Thin marginal seta TMS. White bars = 1 mm, 17e-f 100x, 17g 200x, and 17h 400x.
Figs. 19a-h. Isoperla tilasqua nymph and ventral maxilla, Meacham Creek, Umatilla County, OR. Submarginal setae groups A and B, Marginal setae C, Ventral surface setae D, and Dorsal surface setae DSS. Fig. 5e Inset: Maxillary palp segment 3 with apically rounded long thin setae, 400x. White bars = 1 mm, 19e-f 100x, 19g 200x, and 19h 400x.
| NMNH |
USA, Washington D.C., National Museum of Natural History, [formerly, United States National Museum] |
| TMS |
Toleco Museum of Health and Natural History |
| V |
Royal British Columbia Museum - Herbarium |
| VI |
Mykotektet, National Veterinary Institute |
| R |
Departamento de Geologia, Universidad de Chile |
| CAS |
California Academy of Sciences |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Isoperla baumanni Szczytko & Stewart Isoperla bifurcata Szczytko & Stewart
| Sandberg, John B. 2011 |
Isoperla baumanni
| Isoperla baumanni Szczytko & Stewart 1984 |
Isoperla baumanni
| Isoperla baumanni Szczytko & Stewart 2002 |
Isoperla bifurcata
| Isoperla bifurcata Szczytko & Stewart 1979 |
Isoperla bifurcata:
| Isoperla bifurcata: Bottorff et al. 1990 |
Isoperla baumanni
| Isoperla baumanni Szczytko & Stewart 2002 |
1 (by felipe, 2021-05-14 03:12:35)
2 (by felipe, 2021-05-15 01:21:26)
3 (by felipe, 2021-05-15 01:28:14)
4 (by ExternalLinkService, 2021-05-15 01:28:38)
5 (by ExternalLinkService, 2021-05-15 01:39:50)
6 (by felipe, 2021-05-15 17:25:13)
7 (by ExternalLinkService, 2021-05-15 17:30:27)
8 (by ExternalLinkService, 2021-09-19 03:27:17)
9 (by ExternalLinkService, 2021-10-20 04:03:40)
10 (by felipe, 2023-08-18 16:54:07)
11 (by ExternalLinkService, 2023-08-18 17:05:01)
12 (by plazi, 2023-11-02 10:09:19)