Uperoleia mahonyi, Clulow, Simon, Anstis, Marion, Keogh, J. Scott & Catullo, Renee A., 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4184.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:8A38E47C-5F24-4B2A-A7E8-7D99592252DD |
|
DOI |
https://doi.org/10.5281/zenodo.5618186 |
|
persistent identifier |
https://treatment.plazi.org/id/D15DE063-FFF7-B210-FF47-F9C9FC815520 |
|
treatment provided by |
Plazi |
|
scientific name |
Uperoleia mahonyi |
| status |
sp. nov. |
Uperoleia mahonyi View in CoL sp. nov.
Mahony’s Toadlet
Figs. 3 View FIGURE 3 & 4 View FIGURE 4
Holotype. SAMA R66193 (male), collected in an ephemeral swale on sand at Oyster Cove, NSW (-32.7394, 151.9557) by S. Clulow on 12 February, 2008.
Paratypes. SAMA R66187, SAMA R66188, SAMA R66189, SAMA R66190, SAMA R66191, AMS R185691 and AMS R185692 (adult males), collected at type locality, NSW (-32.7394, 151.9557) on 4 October 2007; SAMA R66192 (adult female), collected at type locality, NSW on 31 March 2007; SAMA R66194 (adult male), collected at the same locality and date as the holotype ; AMS R185695 (adult male), collected at type locality, NSW on 12 October 2009; AMS R185701 (adult female), collected at type locality, NSW on 1 March 2013; SAMA R66186 and SAMA R66195 (sex not determined), collected at type locality, date not recorded; AMS R185693 (adult male), collected in an artificial dam on sand at Nelson Bay Golf Course , NSW (-32.7294, 152.1511) on 5 October 2009 ; AMS R185697 and AMS R185698 (adult males), collected in a sand dune swale behind Stockton Beach , NSW (-32.8293, 151.8825) on 1 November 2009 ; AMS R185696 (adult male), collected in an ephemeral swale on the Tomago sandbed, NSW (-32.7939, 151.7880) on 22 October 2009 ; AMS R185694 (adult male), collected in a Melaleuca swamp off Masonite Road , Tomago, NSW (-32.8026, 151.7646); AMS R185699 and AMS R185700 (adult females), collected in pit traps on a sand dune at Wyrrabalong National Park ~ 400 m from a coastal hind dune swamp (-33.2970, 151.5503) on 28 May 2012 .
Diagnosis. Distinguished as a Uperoleia by a combination of small body size (males 20–30 mm), large parotoid glands covering tympanum, unwebbed fingers, vomerine teeth vestigial or absent, inguinal colouration present, and presence of inner and outer metatarsal tubercles. Distinguished from all other Uperoleia by a combination of ventral pigment (ventral surface completely covered with black and white marbling), presence of maxillary teeth, toes unwebbed, lack of colour patch below the knee, and a “squelch” as a call.
Holotype measurements. Measurements (in mm): SVL—22.2; TibL—9.3; HW—9.0, E—2.6; E-N—1.9; IN—1.7; T—3.3; CP—1.9; CP-K—1.4; CP-V—3.4.
Measurements of series. Mean ± standard deviation in mm. Adult males (n = 11): SVL—25.2±3.1; TibL— 10.0±0.4; HW—9.9±1.1, E—3.1 ±0.6; E-N—2.1±0.3; IN—1.7±0.2; CP—3.2±0.9; CP-K—1.5±0.3; CP-V— 3.7±0.4. Adult females (n = 3): SVL—29.3±2.5; TibL—11.1±1.2; HW—10.7±1.5, E—3.1 ±0.4; E-N—2.3±1.8; IN—1.8±0.2; CP—3.9 (n = 1); CP-K—1.0 (n = 1); CP-V—5.1 (n = 1).
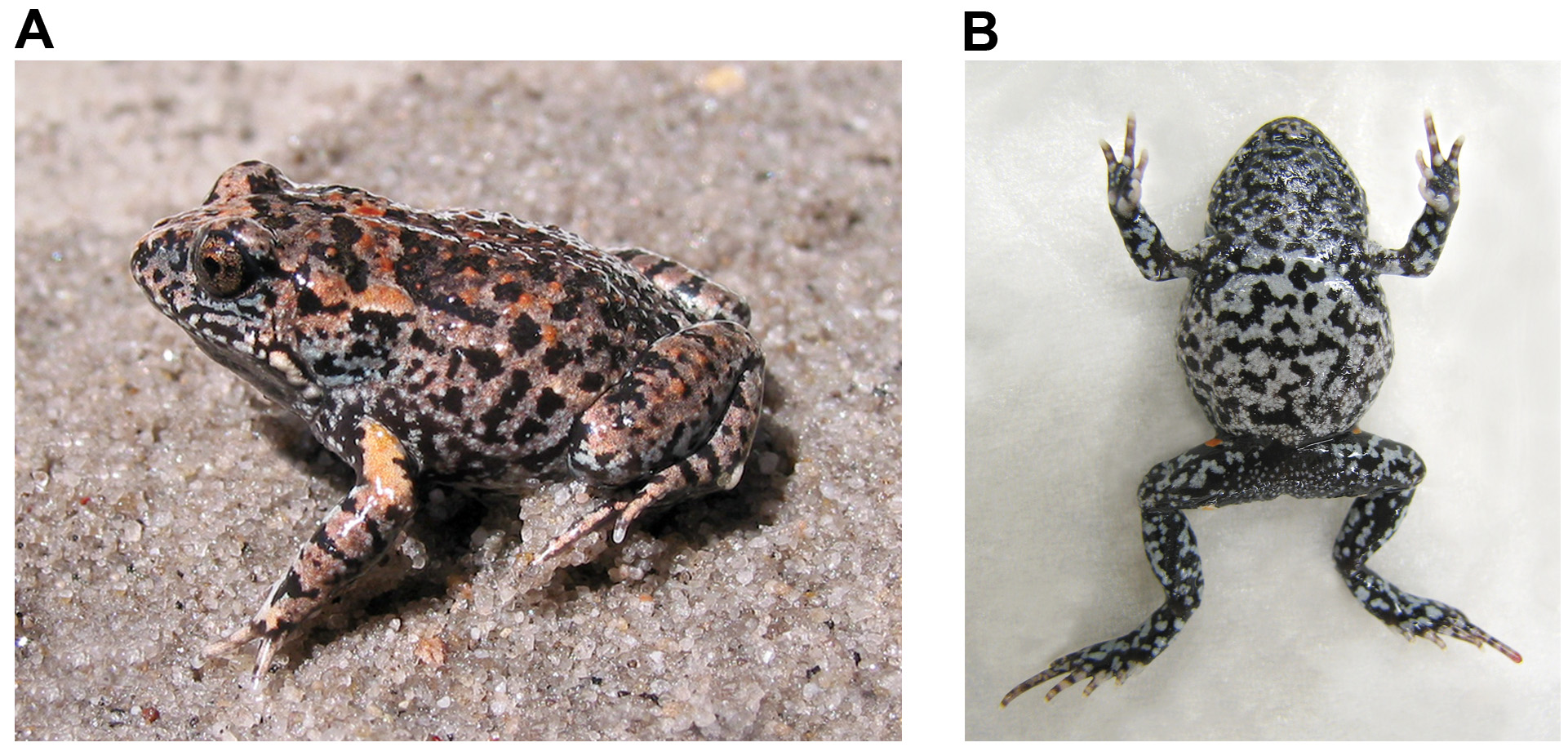
Description of species. Body is robust and moderately large for a Uperoleia , with males up to 30mm SVL and females up to 32 mm SVL. Head is short, snout rounded from above and in profile. Canthus rostralis well defined and slightly protruding; loreal region slopes steeply to jaw and is very slightly concave. There is a moderately sharp medial projection (synthesis of mentomeckelian bones) of the lower jaw that matches notch on upper jaw. Nostrils directed upward and outward; nares with slight rim. Tongue oval and elongate. Maxillary teeth present; vomerine teeth absent. E-N larger than IN (E-N/IN = 1.2 for males and 1.3 for females). Tympana hidden; covered by skin and parotoid glands. Eyes with horizontal iris. Vocal sac unilobular.
Arms and hands slightly built. Fingers long, slender, slightly fringed and unwebbed. Finger length 3>2≥4>1. Tubercles under fingers well developed; one on first and second, two on third and fourth. Well-developed, prominent outer palmar tubercle on distal portion of wrist; well-developed inner palmer tubercle on medial portion of wrist.
Legs relatively short (TL/SVL = 0.4 for both males and females) and moderately built. Toes slender, unwebbed and fringed. Toe length 4>3>5>2>1. Tubercles under toes well developed and slightly conical in shape; one on first and second, two on third and fifth, and three on fourth toe. Inner metatarsal tubercle long and conical, aligned along the first toe. Outer metatarsal tubercle spade-shaped and prominent, oriented in the direction of the fifth toe.
Dorsum smooth to moderately rugose, with scattered fine tubercles on back, head and limbs. Ventral surface weakly granular. Cloacal flap present and fimbriated. Parotoid glands large and prominent, appearing hypertrophied and usually wider than high. Inguinal glands occasionally discernible but not well-developed and rarely obvious. Coccygeal glands indistinct. Mandibular gland moderately developed but small in most, present at corner of the jaw.
Colouration. In life, dorsum patterned with irregular patches of pale, tan, chocolate or dark brown (verging on black) and occasionally greys throughout. In some darker specimens the colour can appear almost uniform. The dorsal colouration usually merges into patterns of bluish grey and dark brown onto the lower flanks. Dorsal tubercles often (but not always) tipped with a pale yellow-orange to rust-orange, which can also occur on the parotoid glands. Many individuals have a lighter brown triangular patch on head from between the eyes to tip of snout, although this can also contain small patterns or flecks of darker brown. Ventral surface entirely pigmented, black with suffusions of irregular patches of small off-white/bluish-white dots. The patches of white dots appear as solid patches to the naked eye, especially on the legs. The patterns of black and white patches appear marbled, more similar to the bellies of Pseudophryne spp . rather than simply stippled as commonly observed in Uperoleia spp . (see Figs 3 View FIGURE 3 & 5 View FIGURE 5 ). Inguinal and femoral colour patches orange in all specimens observed. Femoral colour patch irregular in shape and large and always closer to knee than vent. Throats of calling males may have dark anterior margin, sometimes covering most of the chin.
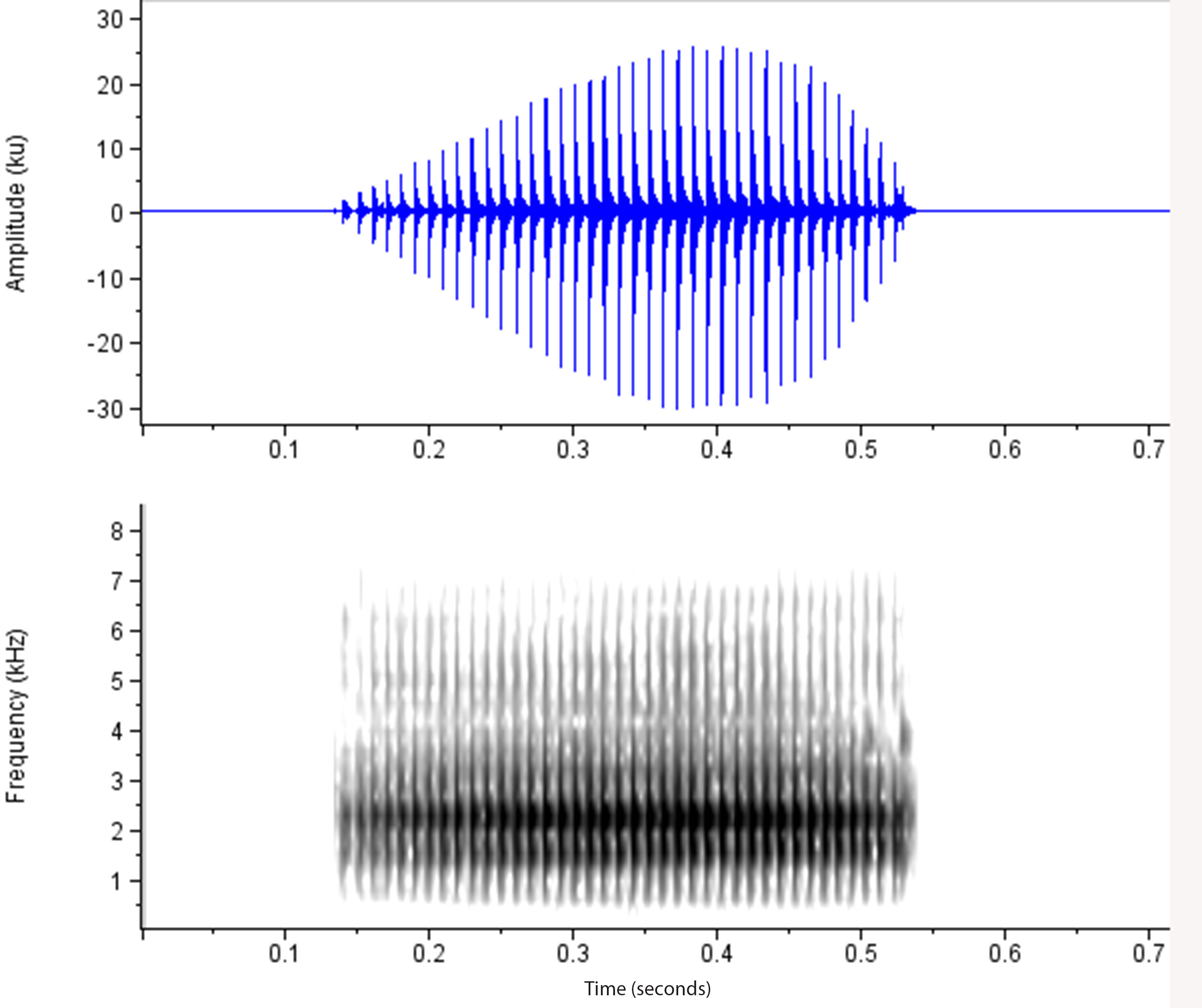
Advertisement call. The advertisement call is a single audible ‘squelch’ sound of about one third of a second duration, repeated on average 25 times per minute (range observed is between 15 and 33 calls per minute from 9 individuals). This ‘squelch’ comprises 24 to 37 pulses, pulsed at 96 pulses per second on average. The mean dominant frequency is 2.37 kHz. Mean values of call characteristics from six individuals from the type locality (over two separate occasions) and one individual from each of three other localities are given in Table 2 View TABLE 2 , along with the call properties of other eastern Uperoleia that are known or potentially occur in sympatry. A representative oscillogram and spectrogram of a single call of Uperoleia mahonyi sp. nov. is presented in Fig. 6 View FIGURE 6. A .
Embryos and tadpoles. Embryos. Breeding is known to occur in autumn (March) and spring (October– November). The total number of eggs laid by one female is unknown. The eggs are laid singly and although only observed in the laboratory and not in the field, under natural conditions they are likely to be attached to thin strands of submerged vegetation and substrate such as leaf litter similar to all other members of this genus ( Anstis, 2013). Eggs laid in the laboratory in autumn and preserved at stages 7–8 were slightly misshapen when examined in 2015, and the jelly had lost some rigidity, but the capsule is small with a single jelly layer and thin, adhesive outer coating, mean diameter 2.8±0.18 (n=8). The top one-third of the animal pole is brown, vegetal pole white. Mean diameter 1.7±0.06 (n=9).
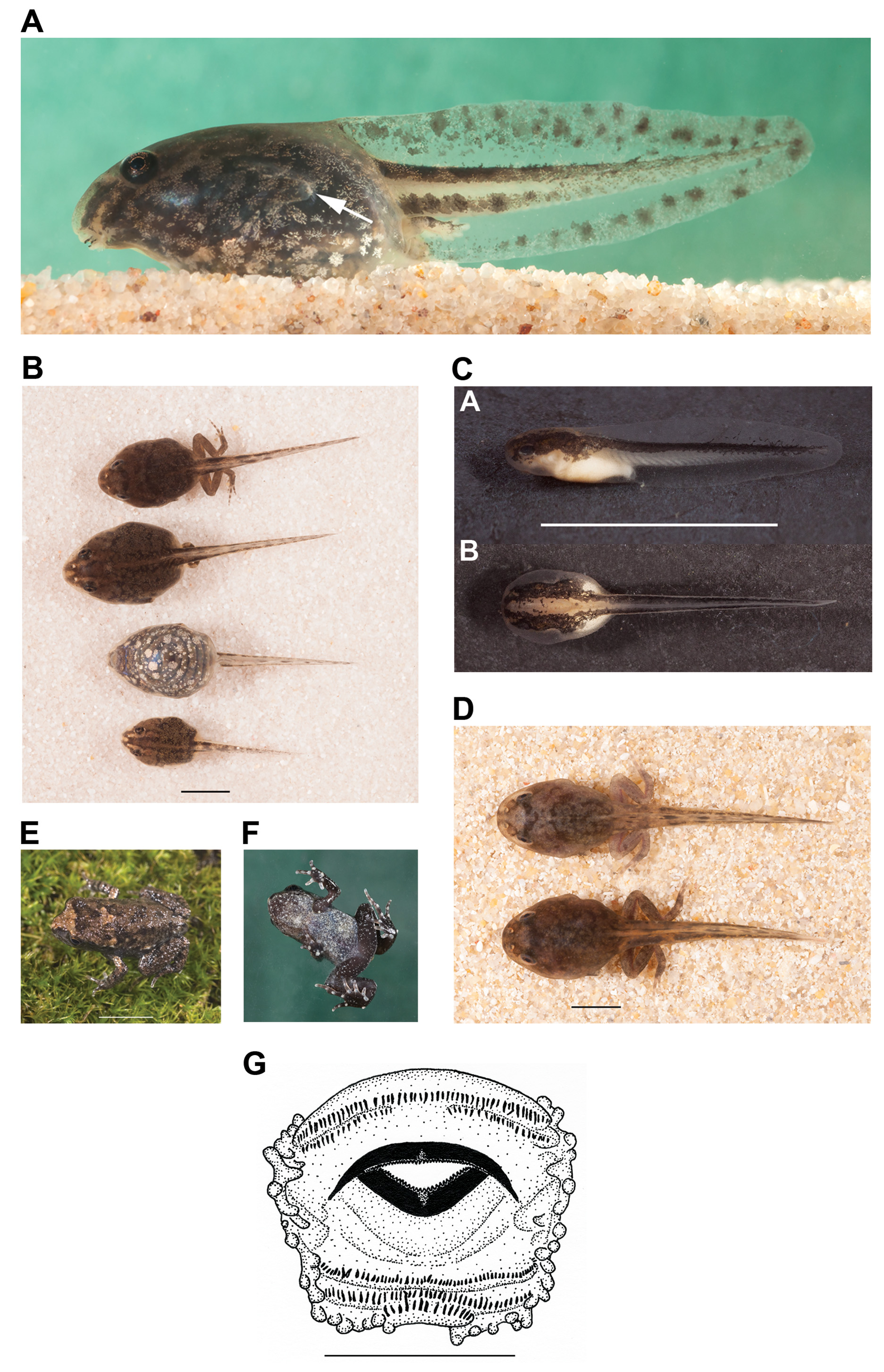
Hatchlings. Hatching occurred 5–6 days after the eggs were laid. One preserved recent hatchling is at stage 20, with brown pigment over head, vertebral region and tail muscle and a white yolk sac, fins not arched. Preserved embryos at stage 22, seven days after eggs were laid, have clear, slightly arched fins, expanded operculum, increased dorsal pigment and discernible, partly pigmented eyes. Mean TL of five hatchlings at stages 20–22 was 7.0±0.48, BL 2.8±0.08. One live embryo at about stage 24 examined about three days after hatching, measured TL 7.1, BL 4.5, Fig. 7 View FIGURE 7 C). The body wall is entirely transparent with an expanded operculum. In lateral view, dorsal one-third of body and tail muscle very dark, yolk white below this and remainder of tail muscle unpigmented. Dorsum and dorsal tail muscle very dark, dissected by a distinct transparent pale brown broad band down centre of body tapering onto tail muscle.
Tadpoles. Tadpoles were found in a large swale at the type locality where they were observed on a sandy substrate among leaf litter, often in the shallow verges of the water. Material from the type locality is listed in Appendix 1 and morphometric measurements in Table 3 View TABLE 3 . Maximum length 35.0 mm, BL 12.4 mm (stage 41, Fig. 7 View FIGURE 7 B). Almost fully grown by stage 28. Figure 7 View FIGURE 7 shows an embryo, tadpoles in life and the oral disc.
Body: Small, plump and oval to rounded, abdomen wider than deep. Snout narrowly rounded in dorsal view, rounded in profile. Eyes dorsolateral with anterodorsal tilt. Nares narrowly spaced, moderately large and cavernous with a narial flap; open dorsally, maximum diameter 0.5mm. Spiracle long, opens lateroposterally just above body axis about two-thirds along body ( Fig. 7 View FIGURE 7 A). Vent tube dextral, very short, opens midway up from edge of ventral fin.
Dorsum of tadpoles at stage 26–30 golden brown to dark brown over almost black layer beneath which shows through in small patches. Lighter brown vertebral stripe bordered on both sides by very dark brown with transparent stripe on either side of this over head, and from between nares to tip of snout. Light brown stripe extends behind each eye. As tadpoles grow, the body is usually dense, dark mottled brown, with the lighter stripes mostly obscured. Iris golden mainly above and below pupil, with gold ring around pupil. Sides of body mostly transparent with numerous gold clusters. Venter transparent with numerous copper-gold clusters, increasing in density in later stages.
Tail: Dorsal fin begins from just onto base of body, arches slightly or moderately over midpoint of tail and tapers to a rounded tip. Ventral fin similarly shaped, but slightly less arched. Muscle moderate, tapers to a narrow point.
A specimen at stage 41 photographed soon after capture has large dark blotches scattered mainly along edges of both fins to tip, and finer melanophores between (especially on dorsal fin), increasing towards tail tip. The tail muscle has a mostly continuous, dark stripe along dorsal and ventral edges (non-pigmented stripe between), with scattered dark blotches. Specimens raised in captivity were similar, but the tail blotches were not as prominent.
Oral disc ( Fig. 7 View FIGURE 7 G): Type 14, ventral ( Anstis, 2013). No papillae around anterior margin. Very narrow posterior medial gap in single row of marginal papillae. Two upper and three lower tooth rows; A2 has a distinct medial gap and P1 has either a very narrow gap or is entire. P3 is the shortest row (about one third length of P2) and sits on edge of flexible ridge. Jaw sheaths slender; upper broadly arched with long sides. LTRF = 2(2)/3(1).
Metamorphosis. Tadpoles collected at stage 41 in autumn metamorphosed six days later. Tadpoles collected at stages 26 and 27 in October and raised in captivity metamorphosed 58 days later in December. Larval life span in spring/summer is therefore likely to be about 3–4 months. One specimen a week after metamorphosis ( Fig. 7 View FIGURE 7 E, F) has a dark brown dorsum with darker spots, a light brown crown on the head and light brown on some tubercles on upper back and on very small parotoid glands. A dark inverted triangular patch mirrors pale crown on head posterior to eyes. Upper arms lighter brown. Sides of body dark grey. Ventral surface of body and limbs whitishgrey with numerous black spots. Ventral surface of a specimen just metamorphosed at stage 46 is dark grey with a dense layer of very fine white spots which are more distinct and spread out on the darker chin and limbs. SVL, 10.1 mm (stage 45), and SVL of another two at stage 46, 10.2 and 13 mm.
Habitat. Current observations indicate the species is a habitat specialist, inhabiting coastal ephemeral and semi-permanent swamps and swales, and occasionally man made dams, in heath or wallum habitats almost exclusively on a substrate of white/leached sand. Commonly associated with acid paperbark swamps. Females have been caught in pit or funnel traps up to 400m away from these water bodies at several localities.
Water bodies containing calling males ranged from ca. 70m x 20 m up to 300m x 500m in size, and from ca. 10–50 cm in depth. Water salinity recorded at two sites ranged up to 0.1 parts per thousand at two water bodies and dissolved oxygen between 4.53 and 6.24 mg /L.
Vegetation communities in which the frog has been found include wallum heath, swamp mahogany-paperbark swamp forest, heath shrubland and Sydney red gum woodland. Terrestrial vegetation associations include the tree species Melaleuca quinquenervia , Eucalyptus robusta , Angophora costata , Acacia longifolia and Banksia spp .
(including B. serrata and B. aemula ). Shrub and herb species include Geebung ( Persoonia lanceolata ), drumsticks ( Isopogon anemonifolius ), heathy parrot pea ( Dillwynia retorta ), bracken ( Pteridium esculentum ), mat rush ( Lomandra longifolia ), heathy Platysace ( Platysace lanceolata ), sweet scented wattle ( Acacia suaveolens ), blady grass ( Imperata cylindrical ), swamp water fern ( Blechnum indicum ), harsh ground fern ( Hypolepis muelleri ), zigzag bog rush ( Schoenus brevifolius ), native rush ( Baloskion pallens ), Leptocarpus tenax and Gahnia clarkei . Aquatic vegetation associations include Shoenoplectus spp ., Baumea spp ., Typha orientalis and Lepironia articulata .
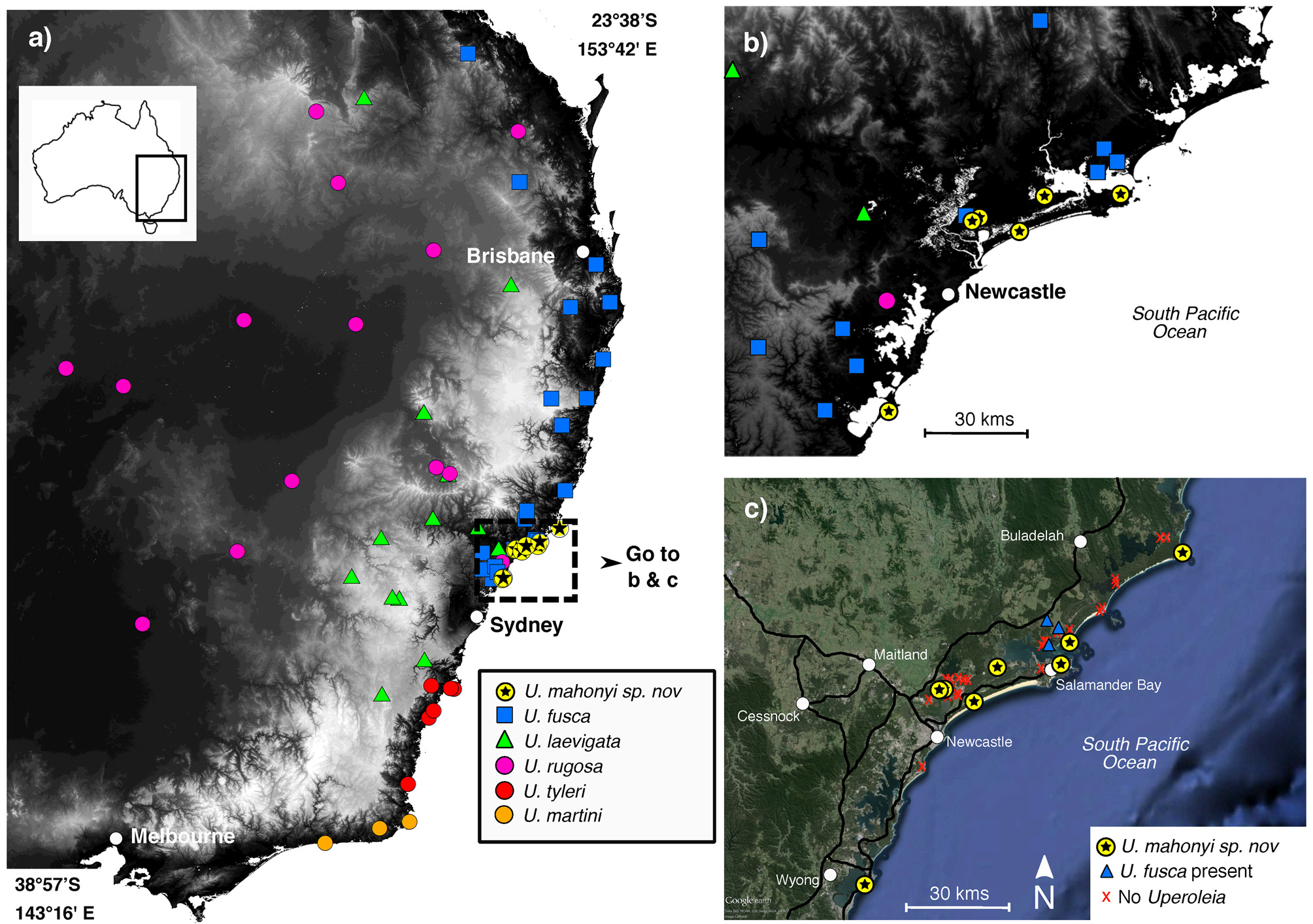
Distribution and frog species associations. The species appears to have a highly restricted distribution, found to date only throughout the Port Stephens, Myall Lakes and northern Central Coast sand beds in a relatively small area of eastern coastal New South Wales ( Fig. 1 View FIGURE 1 ).
A total of 45 sites were surveyed throughout the Port Stephens and Myall Lakes sand bed systems. Six sites in the Port Stephens sand beds were found to contain U. mahonyi sp. nov. in addition to the sites already known at the type locality at Oyster Cove ( Table 4; Figure 1 View FIGURE 1 c). Uperoleia fusca was observed calling in an ephemeral swale <100 m from an area of Melaeuca swamp where U. mahonyi sp. nov. was calling at one site. No sites surveyed in the Myall Lakes system were found to contain U. mahonyi sp. nov. during the formal surveys, although four sites contained U. fusca ( Table 4; Figure 1 View FIGURE 1 c). There were, however, records of U. mahonyi sp. nov. identified from quality photographs obtained from local biologists and enthusiasts in Hawks Nest and Seal Rocks, located at the southern and northern ends of the Myall Lakes sand beds respectively ( Fig. 1 View FIGURE 1 B). Uperoleia mahonyi sp. nov. was also identified from quality photographs at Wyrrabalong National Park and Norah Head on the NSW Central Coast (later confirmed from voucher specimens collected; Fig. 1 View FIGURE 1 b).
At sites where U. mahonyi sp. nov. were located, calling activity was generally high, with estimates of calling males ranging from ca. 6 to>25. All water bodies occupied by U. mahonyi sp. nov. occurred on a substrate of leached (often white) sand.
Fourteen other non- Uperoleia species of frog were found throughout the formal surveys ( Table 4). Eight of these species were found to co-exist in the same water bodies as U. mahonyi sp. nov.
Etymology. Named in recognition of Prof. Michael Mahony of the University of Newcastle, for his contributions to the study of Australian amphibians.
Comparisons with other species. Superficially, U. mahonyi sp. nov. most closely resembles the large, ventrally pigmented eastern U. tyleri and U. martini ; although the ranges of both are geographically separated from U. mahonyi sp. nov. by several hundred kms. It can be distinguished from these and all other Uperoleia by the distinct black and white marbled pattern on the ventral surface of U. mahonyi sp. nov., formed by relatively continuous patches of white dots on a black background. The ventral surfaces of other eastern Uperoleia including U. fusca , U. tyleri and U. martini all present a more even suffusion/stippling of white or off-white pigment on a dark background, which appears more speckled than marbled (refer to Fig. 5 View FIGURE 5 for ventral images of Uperoleia mahonyi sp. nov., U. tyleri and U. martini ). Uperoleia laevigata and U. rugosa both lack ventral pigmentation in at least the groin region and arms (and sometimes much of the belly).
Uperoleia mahony sp. nov. can be further distinguished from U. tyleri by a longer call with more pulses and a higher dominant frequency, from U. martini by a shorter call (almost half the duration) with ca. 50% less pulses, and a higher dominant frequency, and from U. fusca by having more pulses per call ( Table 2 View TABLE 2 ). Uperoleia mahonyi sp. nov. has orange colour in the inguinal and femoral patches in all specimens observed to date, while U. martini and U. tyleri usually have yellow coloured patches.
Tadpoles of all species of eastern Uperoleia can be distinguished from tadpoles of other myobatrachid genera of similar size by a combination of their characteristic blotched tail pigmentation, position of the spiracle, oral disc and larger nares. Tadpoles of Uperoleia mahonyi sp. nov. closely resemble those of other species of Uperoleia and no reliable means of separation of sympatric species was found. They do not appear to grow as large (to 35 mm) as those of other coastal species U. tyleri , U. martini , U. fusca and U. laevigata , all of which can reach a maximum of 42 mm in length and a body length of 15 mm ( Anstis, 2013).
TABLE 3. Morphometric measurements of preserved larval Uperoleia mahonyi sp. nov. from the type locality. Values in mm, mean ± STD with range shown beneath. Developmental stages follow Gosner (1960). Abbreviations for measurements are explained in Table 1.
| N | 2 | 4 1 2 | 1 | 1 | 1 | 3 | 1 |
|---|---|---|---|---|---|---|---|
| STAGE | 25 | 26 27 29 | 30 | 33 | 34 | 35 | 37 |
| TL | 14, 14.5 | 21.4±2.1; 24.5 24.0, 24.0 19.5–24.0 | 24.0 | 26.0 | 26.6 | 26.6±1.7; 25.3–28.5 | 29.2 |
| BL | 6.0, 6.1 | 9.5±1.2; 8.1– 11.3 10.6, 10.8 10.9 | 10.3 | 11.8 | 11.6 | 11.7±0.1; 11.6–11.8 | 13.1 |
| BW | 4.0, 4.2 | 6.5±0.9; 5.3– 7.9 7.4, 7.9 7.4 | 7.0 | 8.2 | 7.9 | 7.9±0.3; 7.6–8.1 | 8.7 |
| BD | 3.1, 3.5 | 5.9±1.2; 4.8– 6.9 6.1, 6.6 7.4 | 5.8 | 7.1 | 6.1 | 6.7±0.1; 6.6–6.8 | 7.6 |
| EBW | 3.2, 3.2 | 4.9±0.6 6.0 4.0, 5.3 4.0–5.3 | 5.3 | 6.1 | 5.8 | 5.7±0.3; 5.5–6.0 | 6.0 |
| IO | 1.0, 1.1 | 1.6±0.2; 1.3– 1.7 1.9, 2.1 1.8 | 1.9 | 2.3 | 1.9 | 2.2±0.4; 1.9–2.6 | 2.3 |
| IN | 0.5, 0.6 | 0.7±0.1; 0.8– 0.8 0.8, 1.0 0.8 | 0.8 | 1.0 | 1.0 | 0.9±0.1; 0.8–1.0 | 0.8 |
| N | 0.2,0.2 | 0.4±0.1 0.2– 0.4 0.4, 0.5 0.5 | 0.4 | 0.5 | 0.4 | 0.5±0.1; 0.4–0.6 | 0.4 |
| EN | 0.5, 0.5 | 0.7±0.1; 0.6– 0.8 0.8, 0.8 0.8 | 0.8 | 0.8 | 0.8 | 0.8±0; 0.8–0.8 | 0.8 |
| SS | 4.7, 4.8 | 7.4±0.1; 6.1– 8.5 8.2, 8.9 8.4 | 7.9 | 9.2 | 9.0 | 9.0±0.5; 8.5–9.4 | 9.5 |
| SN | 0.6, 0.6 | 0.9±0.2; 0.8– 0.8 0.8, 1.0 1.1 | 0.8 | 1.0 | 1.1 | 1.0±0.2; 0.8–1.1 | 1.0 |
| SE | 1.1, 1.1 | 1.9±0.3; 1.5– 1.7 2.1, 2.3 2.1 | 2.1 | 2.1 | 2.3 | 2.2±0.2; 2.1–2.4 | 2.3 |
| ED | 0.6, 0.6 | 1.0±0.1; 0.8– 1.1 1.1, 1.1 1.1 | 1.0 | 1.3 | 1.3 | 1.3±0.1; 1.2–1.3 | 1.5 |
| BTM | 1.1, 1.1 | 1.7±0.2; 1.5– 2.3 1.8, 2.1 1.9 | 1.8 | 2.4 | 2.3 | 2.2±0.3; 1.9–2.4 | 2.3 |
| TD | 3.2, 3.4 | 4.6±0.4; 4.2– 5.6 5.2, 5.4 5.0 | 5.7 | 6.0 | 6.1 | 6.0±0.7; 5.3–6.6 | 5.8 |
| DF | 1.3, 1.5 | 2.0±0.3; 1.6– 2.6 2.3, 2.3 2.2 | 2.4 | 2.3 | 2.6 | 2.5±0.1; 2.4–2.6 | 2.4 |
| TM | 0.8, 1.0 | 1.2±0.1; 1.1– 1.6 1.3, 1.5 1.3 | 1.6 | 1.8 | 1.9 | 1.7±0.3; 1.5–2.1 | 1.6 |
| VF | 1.0, 1.1 | 1.4±0.1; 1.3– 1.5 1.6, 1.6 1.5 | 1.7 | 1.9 | 1.6 | 1.7±0.2; 1.5–1.9 | 1.8 |
| ODW | 1.2, 1.2 | 1.7±0.2; 1.4– 1.0 1.9, 2.0 1.9 | 1.8 | 2.0 | 2.1 | 1.9±0.3; 1.7–2.3 | 2.0 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.