Aequorea neocyanea
|
publication ID |
https://doi.org/10.35929/RSZ.0049 |
|
DOI |
https://doi.org/10.5281/zenodo.5718503 |
|
persistent identifier |
https://treatment.plazi.org/id/D0118A7C-5B31-0055-FED9-FA09FE7E7F0B |
|
treatment provided by |
Felipe |
|
scientific name |
Aequorea neocyanea |
| status |
|
Aequorea neocyanea View in CoL View at ENA new name
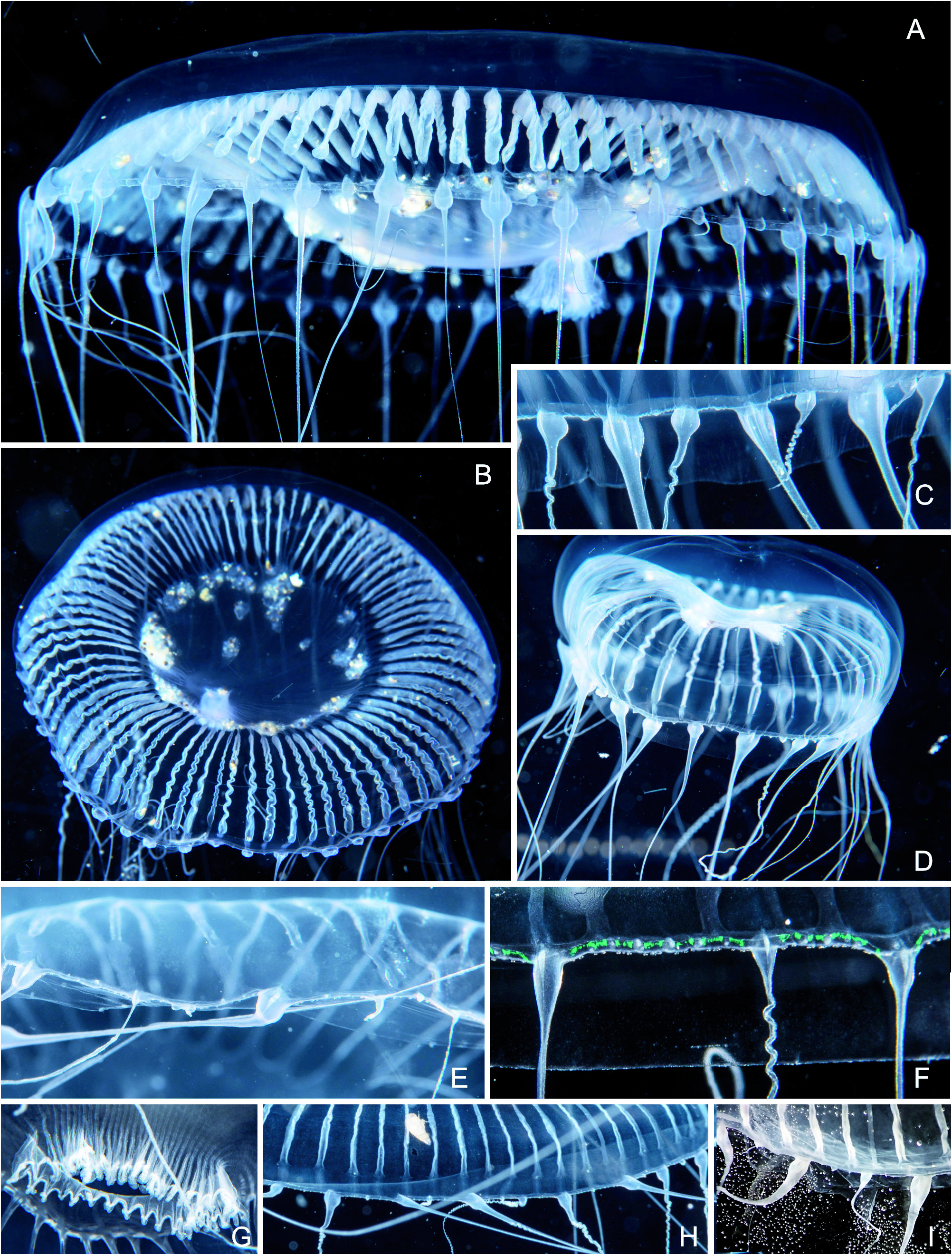
Fig. 38 View Fig A-I
Zygodactyla cyanea L. Agassiz, 1862: 361 View in CoL . [not Aequorea cyanea de Blainville, 1834 View in CoL ]
Zygodactyla cyanea View in CoL . – Agassiz, 1865: 107, fig. 159. – Haeckel, 1879: 227. – Mayer, 1900: 60, pl. 11 fig. 23 & 23a, pl. 15 figs 33-34. – Mayer, 1904: 17, pl. 3 figs 16-17.
in part Aequorea forskalea View in CoL . – Mayer, 1910: 325, Z. cyanea View in CoL as synonym.
? Mesonema coelum-pensile . – Vanhöffen, 1913a: 425, fig. C.
? Aequorea macrodactyla View in CoL . – Nogueira et al., 2016 View Cited Treatment : fig. 1. [not Aequorea macrodactyla ( Brandt, 1835) View in CoL ]
Material examined: BFLA3783 ; 1 specimen; 18-SEP- 2018; size 50 mm, with gonads; part preserved in formalin and deposited as UF-013449 , part in alcohol for DNA extraction; 16S sequence MW528633 View Materials – BFLA3822 ; 1 specimen; 25-OCT-2018; size 40 mm, with gonads; part preserved in formalin and deposited as UF-013427 , part in alcohol for DNA extraction; 16S sequence MW528634 View Materials . – BFLA3827 ; 1 specimen; 14-NOV-2018; size 50 mm, with gonads; part preserved in formalin and deposited as UF-013435 , part in alcohol for DNA extraction; 16S sequence MW528635 View Materials . – BFLA4043 ; 1 specimen; 01-APR-2019; size 50 mm, with gonads; part preserved in formalin and deposited as UF-013436 , part in alcohol for DNA extraction; 16S sequence MW528636 View Materials . – BFLA4082 ; 1 specimen; 07-MAY-2019; size 55 mm, with gonads; part preserved in formalin and deposited as UF-013787 , part in alcohol for DNA extraction; 16S sequence MW528669 View Materials . – BFLA4083 ; 1 specimen; 07-MAY-2019; size 42 mm, with gonads; part preserved in formalin and deposited as UF-013788 , part in alcohol for DNA extraction; 16S sequence MW528670 View Materials . – BFLA4085 ; 1 specimen; 07-MAY-2019; size 60 mm, with gonads; part preserved in formalin and deposited as UF-013789 , part in alcohol for DNA extraction; 16S sequence MW528671 View Materials . – BFLA4236 ; 1 specimen; 21-OCT-2019; size 30 mm, with gonads; part preserved in formalin and deposited as UF-013844 , part in alcohol for DNA extraction; 16S sequence MW528689 View Materials . – BFLA4304 ; 1 specimen; 15-JAN-2020; size 90 mm, with gonads; part preserved in formalin and deposited as UF-013881 , no tissue sample. – 1 specimen photographed 08-FEB-2017, not collected; size 100 mm, with developed gonads. – 1 specimen photographed 06-JUN-2020, not collected; size 70 mm, with developed gonads.
The formalin samples are mostly strongly fragmented and damaged.
Taxonomy: In order to avoid a secondary homonymy with Aequorea cyanea de Blainville, 1834 , we propose here the replacement name Aequorea neocyanea for Zygodactyla cyanea L. Agassiz, 1862 . Zygonema Brandt, 1838 is a synonym of Aequorea Péron & Lesueur, 1810 ( Ranson, 1949) and Agassiz’ Z. cyanea must be transferred to Aequorea .
Observations: Typical Aequorea medusae, diameters of animals with well developed gonads 50 to 100 mm, sizes of animals without gonads (juveniles) up to 30-40 mm. Umbrella in fully grown animals relatively flat ( Fig. 38A View Fig ), about 1/4 of diameter, in younger ones more spherical. Stomach large, diameter 1/2 of bell diameter, with shallow jelly cone inside. Mouth rim with short fimbriae only ( Fig. 38G View Fig ), same number as radial canals, continued centrifugally as fine rib or streak on stomach and then as radial canal. Radial canals in mature animals 25 to 100, more commonly 60 to 80, lower number might also be due to regeneration from fragments. A few (2-4) incomplete radial canals growing centrifugally can be present, also irregularities like fusions or branching, but these likely of traumatic origin. Gonads along radial canals, spanning from almost the beginning to a short distance from circular canal ( Fig. 38 View Fig A-B), bilamellar, when fully developed large and hanging into subumbrella like a curtain, walls much folded or undulated ( Fig. 38A View Fig ). Fully formed tentacles 21 to 50, additionally some small ones or mere bulbs that will likely later also develop into tentacles. Observed ratios of radial canals to fully formed tentacles 1.0-3.0. Tentacles in life nearly always with a swollen base ( Fig. 38C, E, F, H View Fig ) degree of swelling is apparently modifiable and could depend on environment or physiological state as once the animal is preserved the swelling is much reduced ( Fig. 38I View Fig ). Regularly there is a faint abaxial keel, often emphasized or feigned by a whitish line on median of abaxial side ( Fig. 38E, F, H View Fig ) caused by an accumulation of nematocysts, this line only visible in living animals. In swollen bulbs abaxial side or keel often elongated into abaxial spur ( Fig. 38 View Fig A- D), in preserved material much less visible or absent. Excretory papillae absent, excretory pores could not be found reliably in the preserved material. Four or more statocysts (up to 14) between two tentacles or bulbs, 2-3 statoliths per statocyst. Colours: unpigmented, very well grown specimens with a pink hue.
16S data: The eight haplotypes had a range of divergences of 0.3-2.1 % ( Table 1 View Table 1. 16 , intrapopulation variation). A maximum likelihood tree of the partial 16S sequence ( Fig. 37 View Fig ) yielded a diverse but welldefined clade for this species. Its sister clade comprises two samples from the Mediterranean diverging in 3.7- 5.2% of their aligned bases (see discussion below). No relationship to A. forskalea nor to A. macrodactyla is evident though.
Distribution: Florida, Bermuda, perhaps also Brazil and even Mediterranean (see below). Type locality: Atlantic Ocean, USA, Florida, Key West.
Remarks: We think that the present material most likely belongs to the same species identified by Mayer (1900, 1904) as Zygodactyla cyanea , although there are some differences. Zygodactyla cyanea was first described by L. Agassiz (1862) based on animals from Key West, Florida. His brief description was later expanded (A. Agassiz, 1865) and a figure of a fully-grown animal provided. Agassiz (1865) reported it in great numbers along the Florida Reef. Mayer (1900, 1904) then added more details using material from Florida and the Bahamas, notably also figures of the tentacle bulbs and of younger stages. As Mayer was a collaborator of A. Agassiz, his identification was certainly discussed with the latter. In his 1910 monograph, Mayer then synonymized Z. cyanea with Aequorea forskalea Péron & Lesueur, 1810 without further discussion.
Our material matches more Z. cyanea of Mayer (1900, 1904) and not A. forskalea for the following reasons:
1) Mayer found it as very common off the coast of Florida and in our study it was likewise a frequent medusa.
2) The type locality is in the same region and connected by the Gulf stream.
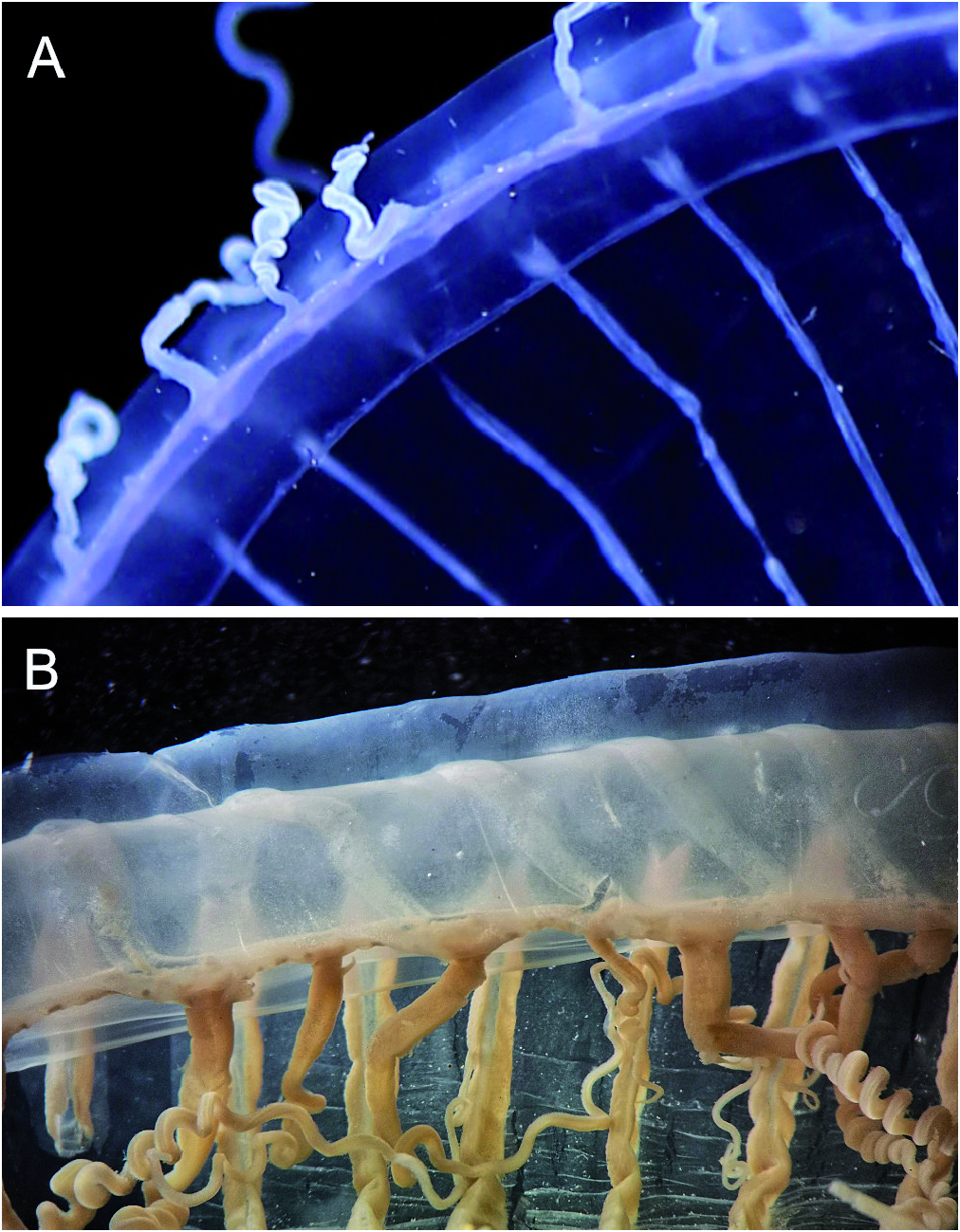
3) Mayer describes and depicts the tentacle bulbs with an abaxial spur, but incorrectly identified it as an exumbrellar excretory papilla (which is unknown in hydromedusae). This corresponds to the bulbs we found ( Fig. 38 View Fig ), although this trait is not a unique diagnostic feature for the species as it occurs also in Aequora spec. 1 (see below) and others, e.g. A. krampi Bouillon, 1984 . Aequorea forskalea in current understanding has evenly tapering, not much swollen tentacle bases ( Fig. 39 View Fig ).
4) The mature animals examined genetically had diameters of 5 to 6 cm, a stomach width of 1/2 the bell diameter, and up to 100 radial canals, thus matching Agassiz’ and Mayer’s values.
There are also traits that do not match. Notably our maximum tentacle number was about 50 and the ratio of radial canals to tentacles usually in the region of 2. Mayer gives up to 100 tentacles and a ratio of 1. These traits are known to be very variable in this genus and should be used with caution to separate species. Moreover, we found that Aequorea medusae often get fragmented and then reconstituted themselves. This vegetative reproduction via fission could account for much of the variation seen in the Aequorea (see Stretch & King, 1980).
Contrary to Mayer (1910), we think that Agassiz’ medusa should be kept distinct from A. forskalea . The 16S sequences of our material were different from A. forskalea of the NE Atlantic ( Fig. 37 View Fig ), the bell sizes were smaller than for typical A. forskalea , and tentacles bases are usually swollen and may have an abaxial keel and spur.
The name Aequorea forskalea was introduced by Péron & Lesueur, 1810 to replace the preoccupied name Medusa aequorea Forsskål, 1775 and they formally also restricted the type locality to the Mediterranean Sea. Forsskål (1775) provided a good illustration of his medusa which he had seen in the NE Atlantic or the Mediterranean and which we must assume to represent the type specimen. Forsskål’s medusa was quite large with a diameter of 23 cm [in his Latin description he states “ Diameter spithamalis ”, a spithame being an ancient Greek/Byzantine length unit corresponding to 0.231 m]. Our current scope of the species was outlined by Russell (1953) and Kramp (1959a) who give sizes of up to 175 mm and 60-80 radial canals. The bases of the tentacles are almost invariably given as evenly tapering and not swollen ( Fig. 39 View Fig , see also Kramp, 1959a: fig. 234b). This is clearly different to the ones observed here ( Fig. 38 View Fig ) but some cautionary remarks are necessary. The degree of inflation of the tentacle base, the keel formation, and the abaxial spur seem to be variable and a partly transient feature. The swelling depends perhaps on the activity of the animal, the osmotic situation, or the digestive cycle. In preserved animals it is much less pronounced ( Fig. 38I View Fig ), but still apparently different from A. forskalea .
The status of the closely related Mediterranean Aequorea samples ( Fig. 37 View Fig , MW528733 View Materials and MW528734 View Materials , see Material & Methods) is not clear. They were immature and 4 to 5 cm in size and their tentacle bases resembled the ones shown in Fig. 38E View Fig and not Fig. 39B View Fig . It could be that they also belong to the present species. The A. forskalea of the Mediterranean also differentiate into two morphotypes when examined alive (unpublished observations): one with slender, evenly tapering tentacles as shown in Fig. 39B View Fig and another with much swollen bases of the bulbs resembling the ones shown in Fig. 38 View Fig A-B.
The Brazilian medusae identified as A. macrodactyla by Nogueira et al. (2016) do not match well the latter species (see Kramp, 1968; Schuchert, 2017a) but conform much better with the scope of A. neocyanea as documented here.
The status of the Mediterranean Aequorea morphotypes as well as many other populations should be examined using genetic techniques.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Aequorea neocyanea
| Schuchert, Peter & Collins, Richard 2021 |
Mesonema coelum-pensile
| Vanhoffen E. 1913: 425 |
Aequorea forskalea
| Mayer A. G. 1910: 325 |
Zygodactyla cyanea
| Mayer A. G. 1904: 17 |
| Mayer A. G. 1900: 60 |
| Haeckel E. 1879: 227 |
| Agassiz A. 1865: 107 |
Zygodactyla cyanea L. Agassiz, 1862: 361
| Agassiz L. 1862: 361 |