Hessemilimax kotulae ( Westerlund, 1883 )
|
publication ID |
https://doi.org/ 10.5252/zoosystema2023v45a11 |
|
publication LSID |
urn:lsid:zoobank.org:pub:454B2701-ABB8-41B7-83B2-51EF4736EC6E |
|
DOI |
https://doi.org/10.5281/zenodo.8120561 |
|
persistent identifier |
https://treatment.plazi.org/id/CC189030-FF99-FFC2-0DD9-2A48D858F86F |
|
treatment provided by |
Felipe |
|
scientific name |
Hessemilimax kotulae ( Westerlund, 1883 ) |
| status |
|
Hessemilimax kotulae ( Westerlund, 1883) View in CoL View at ENA
Vitrina kotulae Westerlund, 1883: 54 View in CoL .
Vitrinopugio kotulae – Hesse 1923: 111.
Semilimax kotulae View in CoL – Forcart 1944: 666.
Semilimax (Hessemilimax) kotulae View in CoL – Schileyko 1986: 134.
Hessemilimax kotulae View in CoL – Giusti et al. 2011: 336, accepted name in MolluscaBase (https://www.molluscabase.org/, consulted on 21.X.2022).
REMARK
Giusti et al. (2011) treated kotulae in a mono-specific genus distinct from Semilimax Stabile, 1859 on the basis of a few anatomical differences including the presence in Hessemilimax of: 1) a long finger-like penile complex; 2) a distinctive terminal portion of the main pilaster; and 3) the absence of a horn-like chitinous structure within the atrial-vaginal stimulator papilla. However, Giusti et al. (2011) recognised that some of their analyses indicated that Hessemilimax constitutes a monophyletic group with the species of Semilimax and that the basal relationships of this clade remain unresolved (see Pfarrer et al. [2021] for a critical discussion and alternative results to the approach of the Giusti et al. [2011]).
ORIGINAL DESCRIPTION
Testa perdepressa, auriformis, tenuissima, virescente-hyalina, superne sub-lente ruguloso-striata; spira plana, 2/5 longitudinis aequans; anfr.2, fortissime accrescentibus, ultimus depressissimus; apertura maxima, fere 7/8 testae longitudinis efficiens, anstrorsum latior, margine columellari fortissime exciso usque ad apicem testae, ut infra conspecta spira tota cum vertice bene conspicua, margine superiore parum exciso, margine anteriore rotundatosubtruncato; limbus membranaceus jam ab anfractu penultimo fere ad marginem anteriorem prolongatus, medio latissimus et fere 1/2 baseos occupans. Long. 5-6, lat. 31/2-4, alt. 2mm.
TYPE LOCALITY. — Hab. Galicia in M. Tatra, 900-2200’ s.m., praecipue in regione alpina , sub lapidibus non rara. English translation: Inhabits Galicia [historical and geographical region extending over what is today south-eastern Poland and western Ukraine] in the Tatra Mountains, 900-2200 m a.s.l., especially in the alpine region, not rare under the stones.
TYPE MATERIAL. — Unknown ( Sysoev & Schileyko 2009).
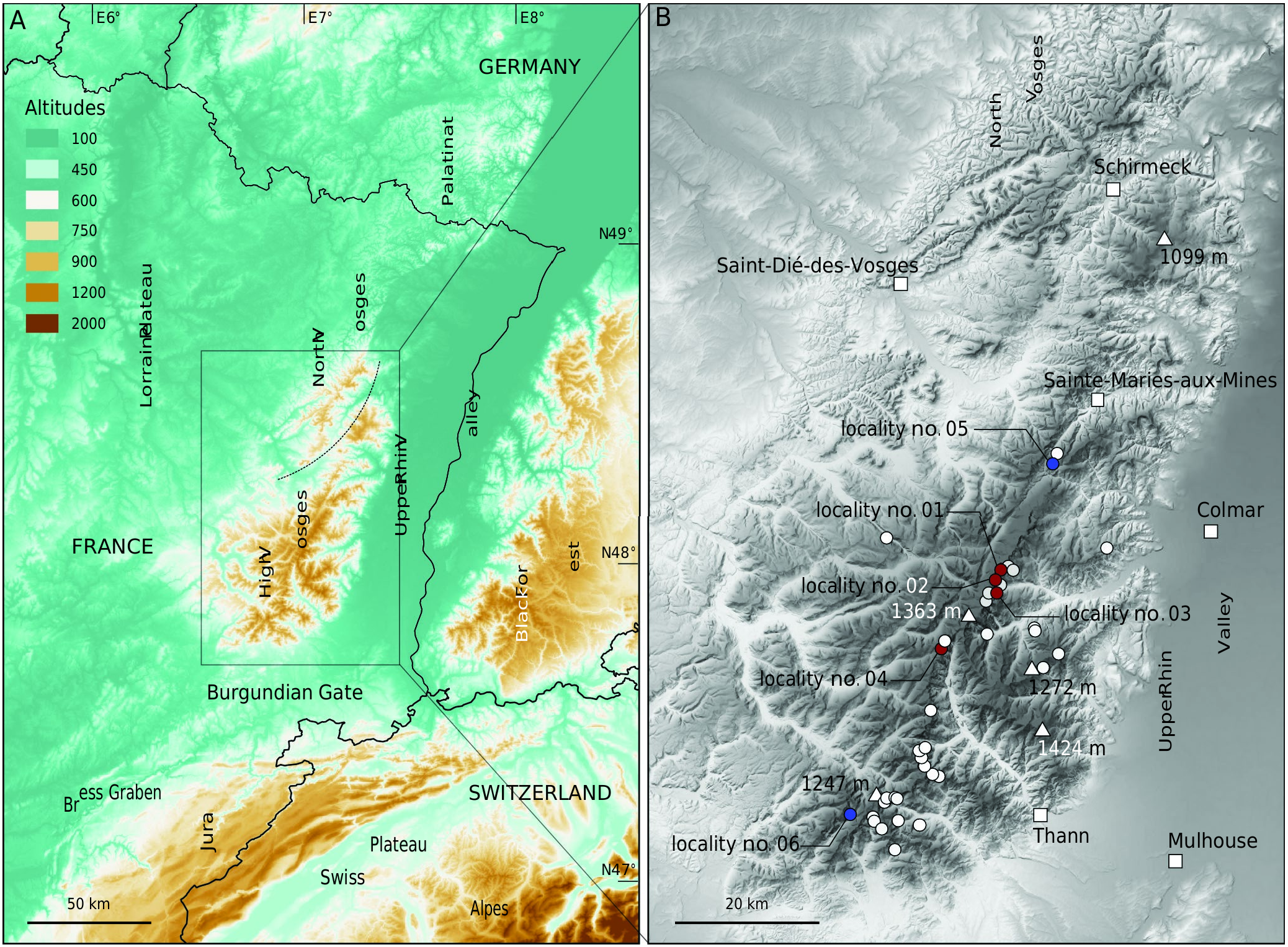
MATERIAL EXAMINED. — France (new records) • 2 live adult specimens; Haut-Rhin , Stosswihr, Hirschsteinried; 48°4’12”N, 7°2’7”E; elevation 1050 m; 08.IX.2022; J.-M. Bichain; MHNEC (locality no. 01 on Fig. 1B View FIG ) GoogleMaps • 1 living juvenile specimen; Haut-Rhin , Stosswihr, Hirschsteinried; 48°04’12.8”N, 7°02’07.7”E; elevation 1050 m; 17.IX.2022; J.-M. Bichain; MHNEC (locality no. 01 on Fig. 1B View FIG ) GoogleMaps • 2 live adult specimens; Haut-Rhin , Stosswihr, Schluchtmatt; 48°3’35”N, 7°1’46”E; elevation 880 m; 29.IX.2022; J.-M. Bichain; MHNEC (locality no. 02 on Fig. 1B View FIG ) GoogleMaps • 1 live adult specimen; Haut-Rhin , Stosswihr, Rothried; 48°2’30”N, 7°1’38”E; elevation 895 m; 26.X.2022; J.-M. Bichain, A. Foltzer & L. Retz; MHNEC (locality no. 03 on Fig. 1B View FIG ) GoogleMaps • 3 live adult specimens; Haut-Rhin , Wildenstein, Pourri-Faing; 47°59’30”N, 6°56’45”E; elevation 1145 m; 31.X.2022; J.-M. Bichain & A. Stoffer; coll. JMB (locality no. 04 on Fig. 1B View FIG ) GoogleMaps .
HABITATS AND SPECIES DIVERSITY
Among the 41 sampling sites, live individuals of Hessemilimax kotulae were found at four, three of which are located within the boundaries of the FM-NNR ( Fig. 1B View FIG ). The locality of Brugel (2014) (locality no. 06 on Fig. 1B View FIG ) did not yield any live specimens nor empty shells of H. kotulae despite an intensive search for several hours. Also, we did not find S. semilimax at the locality indicated by Geissert (1996a) (locality no. 05 on Fig. 1B View FIG ), although we did collect all other species listed by this author.
Hessemilimax kotulae was first collected (two adults and one juvenile, black mantle) in a small swampy area from a small stream in a mixed forest dominated by fir (locality no. 01 on Figs 1B View FIG ; 2A, E View FIG ). The species was also collected (two adults, variegated mantle) at the foot of a scree slope colonized by maple, in particular between moss-covered boulders with underflow (locality no. 02 on Figs 1B View FIG ; 2B, F View FIG ) and at the foot of an avalanche slope (1 adult, variegated mantle) among boulders covered by dense vegetation of nettles and Lunaria sp. (locality no. 03 on Figs 1B View FIG ; 2C, H View FIG ). Finally, the species was observed (three adults, black mantle) in a beech forest, with firs and maples on northeast-facing scree slopes (locality no. 04 on Figs 1B View FIG ; 2D, I View FIG ). This last habitat appears to be rather dry, without perennial surface water runoff. However, this scree slope has many small but deep interstitial spaces that may provide cool and wet refuges for the species. In this habitat, we also found a live specimen of Mediterranea depressa (Sterki, 1880) , a central European species that is also at the westernmost limit of its distribution in the Vosges ( Bichain & Ryelandt 2021). All these localities at which H. kotulae were found are at an elevation between 863 m and 1145 m and are no more than 11 km apart from each other.
The 41 sampling sites yielded 47 gastropod species, including 27 species from the four sites where H. kotulae occurs, with: Acanthinula aculeata (O. F. Müller, 1774) , Aegopinella nitens (Michaud, 1831) , Aegopinella pura (Alder, 1830) , Arianta arbustorum (Linnaeus, 1758) , Clausilia bidentata (StrØm, 1765) , Cochlicopa lubrica (O. F. Müller, 1774) , Cochlodina laminata (Montagu, 1803) , Discus rotundatus (O. F. Müller, 1774) , Edentiella edentula (Draparnaud, 1805) , Eucobresia diaphana (Draparnaud, 1805) , Euconulus fulvus (O. F. Müller, 1774) , Helicodonta obvoluta (O. F. Müller, 1774) , Isognomostoma isognomostomos (Schröter, 1784) , Lehmannia marginata (O. F. Müller, 1774) , Limax cinereoniger Wolf, 1803 , Macrogastra attenuata (Rossmässler, 1835) , Macrogastra plicatula (Draparnaud, 1801) , Malacolimax tenellus (O. F. Müller, 1774) , Mediterranea depressa (Sterki, 1880) , Monachoides incarnatus (O. F. Müller, 1774) , Nesovitrea hammonis (StrØm, 1765) , Oxychilus alliarius (J. S. Miller, 1822) , Oxychilus cellarius (O. F. Müller, 1774) , Phenacolimax major (A. Férussac, 1807) , Trochulus cf. sericeus (Draparnaud, 1801) , Vitrea crystallina (O. F. Müller, 1774) , Vitrina pellucida (O. F. Müller, 1774) . If we include the localities of Brugel (2014) and Geissert (1996a), with the hypothesis that this author’s Semilimax corresponds in fact to S. kotulae , a total of 41 species could potentially occur in syntopy with H. kotulae .
Finally, the regional database ( Bichain et al. 2021) records 79 species in the High Vosges above 600 m elevation (178 sampling sites with 1215 species occurrences, maximum elevation 1320 m, mean elevation 838 m, median 779 m). The data acquired in this study (41 sampling sites with 414 species occurrences, minimum elevation 573 m, maximum elevation 1221 m, mean elevation 926 m, median 940 m) cover 75% of the taxa recorded in the regional database above 800 m (62 spp.) and all taxa known above 1000 m (49 spp.) except for Platyla polita (W. Hartmann, 1840) and Pupilla alpicola (Charpentier, 1837) .
DIAGNOSTIC CHARACTERS
Across the sampled sites, only nine live individuals of Hessemilimax kotulae were observed.According to the criteria given by Umiński (1975), eight specimens were identified as breeding individuals in mature stage II ( Umiński 1975: fig. 14, Figs 2F, H, I View FIG ; 3A View FIG ) and one as a non-breeding individual in juvenile stage III ( Umiński 1975: fig. 12, Figs 2E View FIG ; 3B View FIG ). The description of the reproductive system given below corresponds to the final stage of sexual maturity (mature stage II) sensu Umiński (1975) and Giusti et al. (2011: 336-339, figs 70-73).
Shell morphology and soft body tissue coloration
Shell vitriniform, thin and fragile, transparent, yellow-green, depressed with 1.7 to two whorls; last whorl extremely extended and constituting more than 60% of the overall shell length ( Fig. 2G View FIG ). Protoconch not prominent, smooth without spiral rows of small pits. Very wide aperture with columellar and basal margins bordered by a broad periostracal fringe; umbilicus widely open showing all whorl coils. Shell length of adults: 4-6 mm, shell width of adults: 3-4.5 mm.
Body unable to withdrawn completely into the shell; right mantle lobe long and rather narrow reaching the apex of the shell and covers, especially in juvenile specimens, a large part of the shell when the animal is undisturbed. Body color from black ( Fig. 2E, I View FIG ) to light gray, most often with dark spots giving a variegated pattern ( Fig. 2F, H View FIG ); live adult when extended reaches 15.9 mm ( Fig. 2H View FIG ).
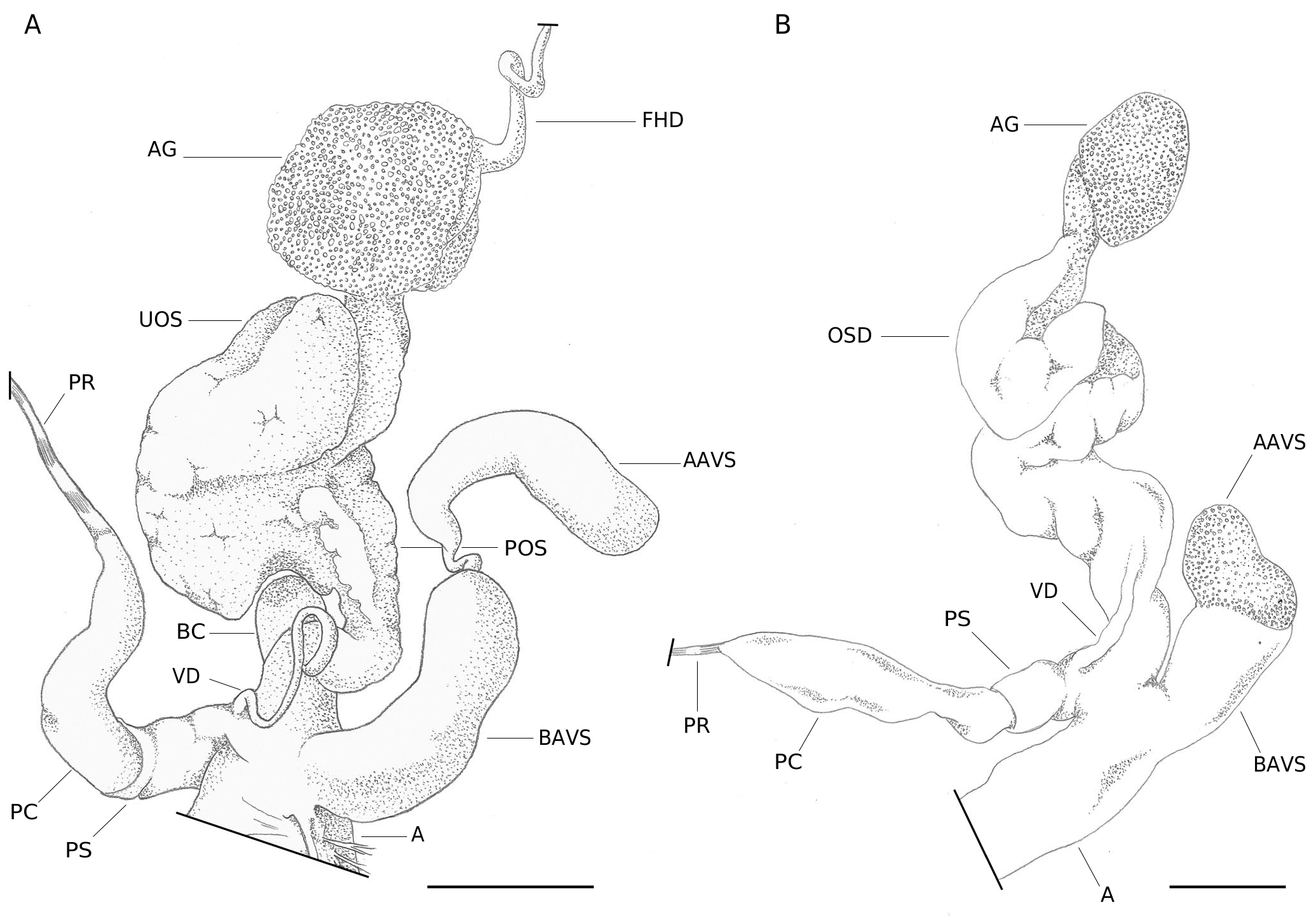
Reproductive system ( Fig. 3A, B View FIG )
Female distal part characterized by short, wide free oviduct; bursa copulatrix with short duct initially slightly flared. Male distal part with large, finger-shaped penial complex; penial gland covering most of the proximal penial complex; penial sheath enveloping almost the entire distal part of the penial complex. Penial retractor long, inserted apically near the emergence of the vas deferens. Vas deferens long, crossing penial sheath, running along the surface of the free oviduct and entering its distal part before ovispermiduct. Internal structure of the penial complex consisting in two distinct pilasters (see Giusti et al. 2011: fig. 72). Main pilaster ending in a π-shaped papilla; smaller pilaster appearing next to the main pilaster and ending about halfway along its length.
Wide and long atrial-vaginal stimulator inserted on the opposite side of the penial complex. Basal part of the atrial-vaginal stimulator sac-shaped without external glandular coating. Apical part with a slender portion more or less invaginated into the basal part of the atrial-vaginal stimulator while the free sac-like part is covered with a spongy external glandular coating. Internal papilla of stimulator short to long, conical, pointed, open at tip, protruding into basal part of the atrial-vaginal stimulator (see Giusti et al. 2011: fig. 73).
REMARK
According to Umiński (1975), the juvenile stage III is achieved when individuals reach a shell diameter of 2.6-3.7 mm and genitalia length of 3.1-4.1 mm. Changes between the juvenile stage III and the mature stage I are mainly quantitative, with the increase in size of the different parts of the reproductive system. However, the emergence of glandular tissue covering the proximal part of the penial complex seems to determine the onset of the mature stage I. Based on these criteria, we assume that the specimen presented in Figures 2E View FIG and 3B View FIG is probably a juvenile stage III.
MORPHOLOGICAL AND ANATOMICAL DIFFERENCES FROM OTHER SYMPATRIC VITRINID SPECIES
Three other vitrinid species were sampled in sympatry or in syntopy with H. kotulae : Eucobresia diaphana ( Fig. 4A View FIG ), Phenacolimax major ( Fig. 4B View FIG ) and Vitrina pellucida ( Fig. 4C View FIG ). Anatomically, H. kotulae is easily distinguishable by the presence of an atrial-vaginal stimulator, by its flat and enlarged shell with only two whorls and an umbilicus widely open. In addition, the live animal often has a variegated mantle, an appearance absent in the other species. Vitrina pellucida has a much more globular shell with three whorls and a pale body. Phenacolimax major has a flatter shell than V. pellucida also with three whorls but a darker body. In these two species, the lobe of the mantle does not reach the apex of the shell ( Fig. 4B, C View FIG ) except when the animal is more or less stressed. Eucobresia diaphana has a flatted and elongated shell with 2.5 whorls but the last whorl is less developed than in H. kotulae and has a much less open umbilicus. The body can be light grey or entirely black but never with a variegated pattern like H. kotulae . Also, the lobe covering the apex is spatula-shaped and wider than in H. kotulae . However, some specimens in the field, particularly juveniles of H. kotulae , may be more difficult to differentiate from E. diaphana and require more careful observation or even examination of the genitalia.
Finally, all these species, except H. kotulae ( Fig. 4D View FIG ), present spiral rows of small pits on the apex ( Fig. 4E, F, G View FIG ), clearly visible under high magnification of about × 40-80.
PROPOSAL FOR NATIONAL AND REGIONAL IUCN STATUS The mountain glass snail is currently listed in the French IUCN Red List ( UICN comité français, OFB & MNHN 2021) as Near Threatened [NT nr B1a]. However, we argue that the putative effects of global warming on this species ( Müller et al. 2009; Bässler et al. 2010) allow application of option b (continuing decline) of criterion B (geographic range), which implies an estimated, inferred, or projected continuing decline in the: 1) extent of occurrence; 2) area of occupancy; 3) area, extent, and/or quality of habitat; 4) number of sites or subpopulations; and 5) number of mature individuals ( IUCN 2022). Indeed, based on two climate warming scenarios (+1.8°C vs +4.0°C), the statistical models used by Müller et al. (2009) and Bässler et al. (2010) predict a considerable risk of extinction for H. kotulae within the Bavarian Forest National Park, a low mountain range in southeast Germany (elevations <1430 m). Their results suggest that an increase in mean annual temperature of + 1.8°C will lead to a decrease in the probability of occurrence of H. kotulae by about 70% at elevations of 1400 m and that the +4.0°C scenario would probably lead to regional extinction. Consequently, Bässler et al. (2010) speculated that the mountain glass snail is a species highly vulnerable to climate change throughout its geographic range, with a high risk of global extinction.
Currently, H. kotulae is formally documented from France only in the Vosges Mountains; its presence in the Massif Central is speculative and based on old data ( Bouillet 1836; Van Bruggen 1957; Falkner et al. 2002: note 225). Recent field surveys (Sylvain Vrignaud, personal communication), especially in historical localities, have not confirmed its occurrence in the Massif Central. Therefore, for the national and regional IUCN assessment, we rely only on recent data ( Brugel 2014 and this work). To minimize the effects of under-sampling, the maximum area of occurrence (EOO, B1 criterion) is estimated at a maximum of 3000 km 2 (<20 000 km2), i.e., the total area of the central and southern Vosges mountains ( Heuacker et al. 2015), including the High Vosges; the maximum area of occupancy (AOO, B2 criterion) is estimated at a maximum of about 883 km 2 (<2000 km 2), i.e., the total area above 800 m within the EOO. Strict application of the IUCN criteria ( IUCN 2022) based on the number of recently-documented localities where the species occurs (i.e., five), as well as the high degree of fragmentation of suitable habitats (i.e., option a) would lead, with application of option b (see above), to the category Vulnerable [VU B1ab(i, ii, iii, iv, v)+ 2ab(i, ii, iii, iv, v)] at the national scale.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Hessemilimax kotulae ( Westerlund, 1883 )
| Bichain, Jean-Michel & Ryelandt, Julien 2023 |
Hessemilimax kotulae
| GIUSTI F. & FIORENTINO V. & BENOCCI A. & MANGANELLI G. 2011: 336 |
Semilimax (Hessemilimax) kotulae
| SCHILEYKO A. A. 1986: 134 |
Semilimax kotulae
| FORCART L. 1944: 666 |
Vitrinopugio kotulae
| HESSE P. 1923: 111 |
Vitrina kotulae
| WESTERLUND C. A. 1883: 54 |