Anacampsis wikeri Harrison
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3741.1.8 |
|
publication LSID |
lsid:zoobank.org:pub:F2FC03C7-40A2-4E6F-8519-0C876AAEFA99 |
|
DOI |
https://doi.org/10.5281/zenodo.6149394 |
|
persistent identifier |
https://treatment.plazi.org/id/CA5687E0-FF8C-FFFE-FF59-F945FEA5FD80 |
|
treatment provided by |
Plazi |
|
scientific name |
Anacampsis wikeri Harrison |
| status |
sp. nov. |
Anacampsis wikeri Harrison View in CoL , new species
( Figs. 2 View FIGURES 1 – 2 , 4 View FIGURES 3 – 4 , 6 View FIGURES 5 – 6 , 7–10 View FIGURES 7 – 10 )
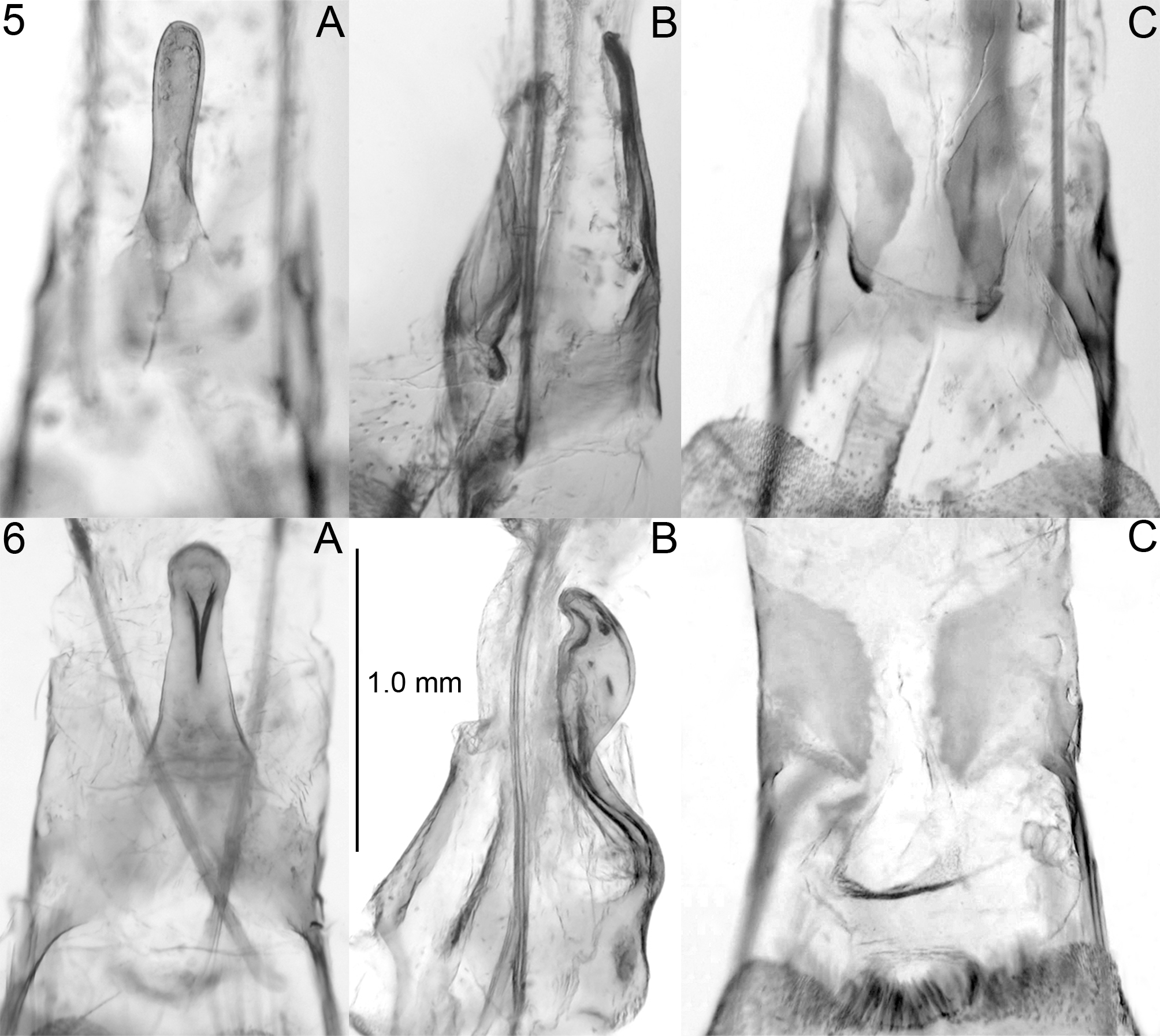
Diagnosis. The adult of A. psoraliella ( Fig. 1 View FIGURES 1 – 2 ) is externally similar to that of A. wikeri ( Fig. 2 View FIGURES 1 – 2 ), but the two species display extensive differences in genital morphology in both genders. In the male genitalia of A. psoraliella , ( Fig. 3 View FIGURES 3 – 4 ), the valva in lateral aspect is narrowest at midlength, then broadens slightly before abruptly narrowing into a spikelike apical projection; the juxta lies in approximately the same dorsoventral plane as the valva and is laterally constricted near its anterior end, its broad posterior region in ventral aspect appearing quadrate, with the posterior margin entire; the vinculum is relatively broad and U-shaped, its length exceeded by the base of the phallus; and the phallus in lateral aspect is relatively narrow in the apical half of its length, with the apex acuminate and the lateral keel running closer to the ventral than to the dorsal margin. In A. wikeri ( Fig. 4 View FIGURES 3 – 4 ), the valva in lateral aspect is relatively narrow along its entire length, its apex rounded; the juxta lies well dorsad of the valva and is without an anterior constriction; it narrows gradually until near the posterior extremity, where it narrows slightly but abruptly into a pair of posterior lobes that are separated by a prominent V-shaped excavation; the vinculum is relatively narrow and V-shaped, its length exceeding the base of the phallus; and the phallus in lateral aspect is relatively broad in the apical half of its length, with the apex truncate and the lateral keel running equidistantly between the dorsal and ventral margins. In the female genitalia of A. psoraliella ( Fig. 5 View FIGURES 5 – 6 ), the tergal projection of A8 is straight and dorsoventrally flattened and narrow along its entire length. In A. wikeri ( Fig. 6 View FIGURES 5 – 6 ), the tergal projection of A8 is doubly curved and not flattened dorsoventrally and is prominently enlarged at its posterior end.
Anacampsis psoraliella also differs from A. wikeri in larval foodplant preference. So far as is known, the larva of A. psoraliella feeds only on plants that formerly were assigned to the genus Psoralea . Specimens of A. psoraliella in the collection of the USNM were reared in Iowa from silverleaf Indian breadroot, Pediomelum argophyllum (Pursh) J. Grimes (labeled as Psoralea argophylla ), in Nebraska from slimflower scurfpea, Psoralidium tenuiflorum (Pursh) Rydberg (labeled as Psoralea tenuiflora ), and in Arkansas from Sampson's snakeroot, Orbexilum pedunculatum (P. Miller) Rydberg (labeled as Psoralea psoralioides ). Emergence dates of these moths are concentrated in the period extending from mid-June through early July, which is slightly later than for A. wikeri in central Illinois. Psoralidium tenuiflorum is common at a number of the Illinois prairies that we surveyed for microlepidoptera (these sites lying along a 240-km, northeast-to-southwest line extending from Mason through Jersey counties), but no larval evidence of A. psoraliella was seen on P. tenuiflorum at these sites, nor were adults taken in our light traps. This could indicate that A. psoraliella is absent from Illinois, but a more extensive survey (including inspection in southern Illinois of Orbexilum pedunculatum , which does not occur on any of the prairies that we surveyed) would be necessary to assess the true status of this species within the state.
Adult ( Fig. 2 View FIGURES 1 – 2 ). Head: Smoothly scaled, brown; antenna brown, basal one third of each flagellomere slightly darker than apical two-thirds; labial palpus brown laterally, paler and more shining brown on inner surface; maxillary palpus greatly reduced; haustellum with pale-brown scales.
Thorax: Collar, tegulae, and dorsum of thorax brown; forewing length 7.2–8.0 mm (n = 15); forewing brown, ground color somewhat paler than dorsum of head and thorax, with a dark-brown patch along CuP fold at 0.2 length present or absent; a few dark-brown scales above fold from base near 0.6, near a diffuse dark-brown transverse fascia at 0.7; a distal, ochreous band formed from a larger anterior and smaller posterior patch; 5–6 small dark-brown spots beyond anterior ochreous patch, extending to apical margin; fringe pale brown, with a narrow, darker band near base; hindwing brown, fringe as above; ventral surface of forewing and hindwing shining brown, slightly paler than dorsum; ventral surface of thorax shining pale brown; all legs with coxa, trochanter, and femur brown, tibia and tarsus concolorous with thorax ventrally, darker and duller brown dorsally.
Abdomen: Brown, darker dorsally than ventrally. Male genitalia ( Fig. 4 View FIGURES 3 – 4 ). Valva straight, subcylindrical, length approximately 7.0X width, exceeding combined vinculum/juxta by approximately 1.1X its length; vinculum Vshaped; juxta with ventroposterior margin U-shaped, posterolateral margin produced into a sharp point; apicolateral lobes prominent, at a 45° angle, with no ventral excavation between lobes; gnathos a transverse band with no ventrally-projecting medial process; phallobase approximately 2.3X as long as wide, about 0.45 length of phallus, distal part of phallus slightly narrower than phallobase, with narrow lateral ridge, broadly curved to a truncate apex. Female genitalia ( Figs. 5-8 View FIGURES 5 – 6 View FIGURES 7 – 10 ). Papillae anales membranous; posterior apophysis narrow, extending anterad approximately to posterior margin of 7th segment; tergite of segment 8 with middorsal posteriorlyprojecting process, curved ventrally in basal 0.45 length, curving dorsad and enlarging abruptly into a broad knob, apical half narrowing to form a slightly curved, thornlike apical process; anterior margin of 8th tergite a broad band from middorsal projection to base of anterior apophysis; anterior apophysis slightly thicker than posterior apophysis and approximately 0.6 shorter, its base merging ventrally into 8th sternite; ostium bursa marked by a broad midventral invagination; ductus bursae narrow and membranous, equal in length to corpus bursae; corpus bursae oval, spiculate, 1.4X as long as wide; signum a narrow transverse band, posterior margin with microserrations, bisecting a finely spiculate area in posterior third of corpus bursae; ductus seminalis about half way between signum and anterior margin of circular area, 0.75X as long as width of corpus, connected apically to an appendix bursae 0.5X as long as corpus bursae.
Mature larva ( Fig. 9 View FIGURES 7 – 10 ). Length, 11.5 mm; cuticle spiculate; head, legs, and prothoracic and anal shield black; small but prominent black sclerotized pinacula present at bases of most of the primary setae or setal groups, especially SD, L, and SV; pinacula larger on T1 and T2 than on other segments; SD1 on A9 hairlike.
Life history. The species is univoltine. At the type locality in central Illinois, mating and oviposition are presumed to occur in early May. The larva feeds on leaves of leadplant, Amorpha canescens . It lives and feeds inside a shelter that it constructs by silking together two or more leaflets at the apex of a leaf, which at the time is in the incipient stage of expanding. This renders the larval shelter difficult to distinguish from the normal growth form of the apical leaflets ( Fig. 10 View FIGURES 7 – 10 ), but sometimes the presence of a larva is indicated by a small area of browning and/ or a few pellets of frass at the apex of the shelter. The larva of Hystrichophora taleana (Grote) (Tortricidae) is present at the same time as A. wikeri and occupies the same feeding mode on leadplant, but H. taleana is relatively stouter and has larger, more visibly prominent setal pinacula than A. wikeri . The larva of A. wikeri matures during the third week of May, after which it pupates and, in early to mid-June, emerges as an adult, in which stage it overwinters.
Host plant and habitat specificity. Three species of Amorpha occur in Illinois. One of them, Amorpha nitens Boynton , is known only from a few isolated localities (none of them prairies) in far southern Illinois (Mohlenbrock and Voigt 1959; Taft 2005) and is listed as an endangered species in the state (Herkert and Ebinger 2002). Not surprisingly, the Lepidoptera fauna (if any) that is associated with this plant in Illinois is not known at present. The remaining two Amorpha species in Illinois are widespread throughout the state (Mohlenbrock and Ladd 1978), but they occupy different habitats: A. canescens is a prairie-restricted plant, whereas Amorpha fruticosa Linnaeus prefers wet areas (Jones 1945). Extensive searching of Illinois populations of A. fruticosa by J. R. Wiker during the past ten years, as part of a survey to document the occurrence of the elachistid moth, Agonopterix dimorphella Clarke , has not yielded any Anacampsis larvae. Therefore, it is concluded that A. wikeri feeds only on leadplant and thus is obligately restricted to prairie habitat.
Distribution. At present, A. wikeri is known only from Illinois hill prairies in Mason and Jersey Counties, and from Allamakee County, Iowa (MJ Hatfield, in litt.). It is hoped that the present paper will encourage surveys for this moth in other areas where leadplant occurs.
Material examined. Holotype ♂. Locality label 1 (white; forward slashes indicate line breaks): “ILLINOIS, Mason County/ Revis Hill Prairie/ T20N-R7W, Section 36/ May 18, 2005 -Larva/ Collected by James R. Wiker”; locality label 2 (white): “Larva found on leaves of/ Amorpha canescens / May 18, 2005 -J. Wiker/ Pupated: Late May, 2005/ Emerged: June 7, 2005 ”; determination label (red): “ HOLOTYPE / Anacampsis wikeri ♂/ Harrison, 2013” (USNM). Paratypes. Four ♂, same locality label as for holotype except (a) collected May 18 2005, emerged June 6, 2005; (b) collected May 18, 2005, emerged June 9, 2005; (c) collected May 25, 2006, emerged June 20, 2006; (d) collected May 17, 2004, emerged mid-June, 2004; six ♀, same locality label as for holotype except (a) collected May 26, 2002, emerged June 18, 2002; (b) collected May 18, 2005, emerged June 11, 2005; (c) collected May 26, 2002, emerged June 14, 2002; (d, e, f) collected May 26, 2006, emerged June 20, 2006; one ♂, locality label (white): “Collected as larva on Amorpha / canescens , USA: Illinois,/ Mason County, Revis Hill/ Prairie, +40° 8' 50.72", -89° 50'/ 24.99", 18-V-2006, T./ Harrison, emerged 6-VI-2006.”; one ♀, same locality label as for previous specimen, except emerged 7-VI-2006; all paratypes with determination label (blue): PARATYPE / Anacampsis wikeri [gender symbol]/Harrison, 2013; paratypes deposited into USNM and collection of James R. Wiker, Greenview, Illinois, USA. Not included in type series. Three ♂, locality label (white): “Collected as adult at UV light,/ USA: Illinois, Jersey County, / Pere Marquette Lost Hill/ Prairie, +39o 0' 58.56", -90o 32'/ 7.42", 23-VI- 2004, T./ Harrison.”; eight ♀, two ♂, same locality label as for previous specimens except collected 14-VI-2005; four ♀, same locality label as for previous specimens except collected 6-VI-2005; four ♀, same locality label as for previous specimens except collected 29-VI-2005; all with determination label (white): “ GELECHIIDAE :/ Anacampsis wikeri [gender symbol]/ Harrison 2013/ Det. T. Harrison, 2013” (USNM).
Etymology. Named in honor of Illinois lepidopterist James R. Wiker, who first reared this species and brought it to the attention of the authors, and who provided much incidental information that was relevant to the description of this moth.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |