Atractus snethlageae
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3721.5.2 |
|
publication LSID |
lsid:zoobank.org:pub:5E654B97-1FD1-4048-BEF2-02911CA5DDFC |
|
DOI |
https://doi.org/10.5281/zenodo.5631981 |
|
persistent identifier |
https://treatment.plazi.org/id/C70787E9-FFA0-664B-FF07-6DE5FB24244B |
|
treatment provided by |
Plazi |
|
scientific name |
Atractus snethlageae |
| status |
|
Atractus snethlageae Cunha & Nascimento 1983
Figs. 1 View FIGURE 1 and 3 View FIGURE 3
Atractus badius (F. Boie) — Amaral 1930a: 93 (in part), 1930b: 59 (in part); Carrillo & Icochea 1995: 13; Doan & Arizábal 2000: 116.
Atractus major Boulenger — Savage 1960: 47 (in part, see remarks); Duellman 1978: 229 (in part, see remarks); Pérez-Santos & Moreno 1991: 94 (in part, see remarks)
Atractus sp. B — Dixon & Soini 1977: 37.
Atractus flammigerus (F. Boie) — Hoogmoed 1980: 20 (in part, specimen from Peru); Dixon & Soini 1986: 93; Duellman & Salas 1991: 9; Duellman 2005: 366.
Atractus flammigerus snethlageae Cunha & Nascimento 1983: 19.
Atractus snethlageae— Vanzolini 1986: 25; Martins & Oliveira 1993: 34; Martins & Oliveira 1999: 97; Giraudo & Scrocchi 2000: 82.
Holotype and type locality. Adult male, MPEG 10131, collected in October 0 3, 1976, at locality of Colônia Nova (01º26’S, 47º32’W), Rio Gurupi, Rodovia BR-316, State of Pará, Brazil.
Diagnosis. A species of Atractus with 17 dorsal scales rows, differing from all congeners by the following combination of characters: (1) large size, adults reaching about 700 mm in TL; (2) loreal long (about three times longer than high); (3) generally seven (rarely eight) supralabials with third and fourth (fourth and fifth whenever eight supralabials are present) in contact with eye; (4) generally eight (rarely seven) infralabials with first four in contact with chinshields; (5) six to seven maxillary teeth; (6) 151–180 ventral scales in females and 137–165 in males; (7) 19–28 subcaudal scales in females and 27–45 in females; (8) tail of moderate length: Tail/TL 6.9– 17.6%; (9) dorsal color pattern of dark gray/brown ground coloration with pale crossbands or blotches usually edged in black, or dorsal ground color pale brown with dark brown or black blotches.
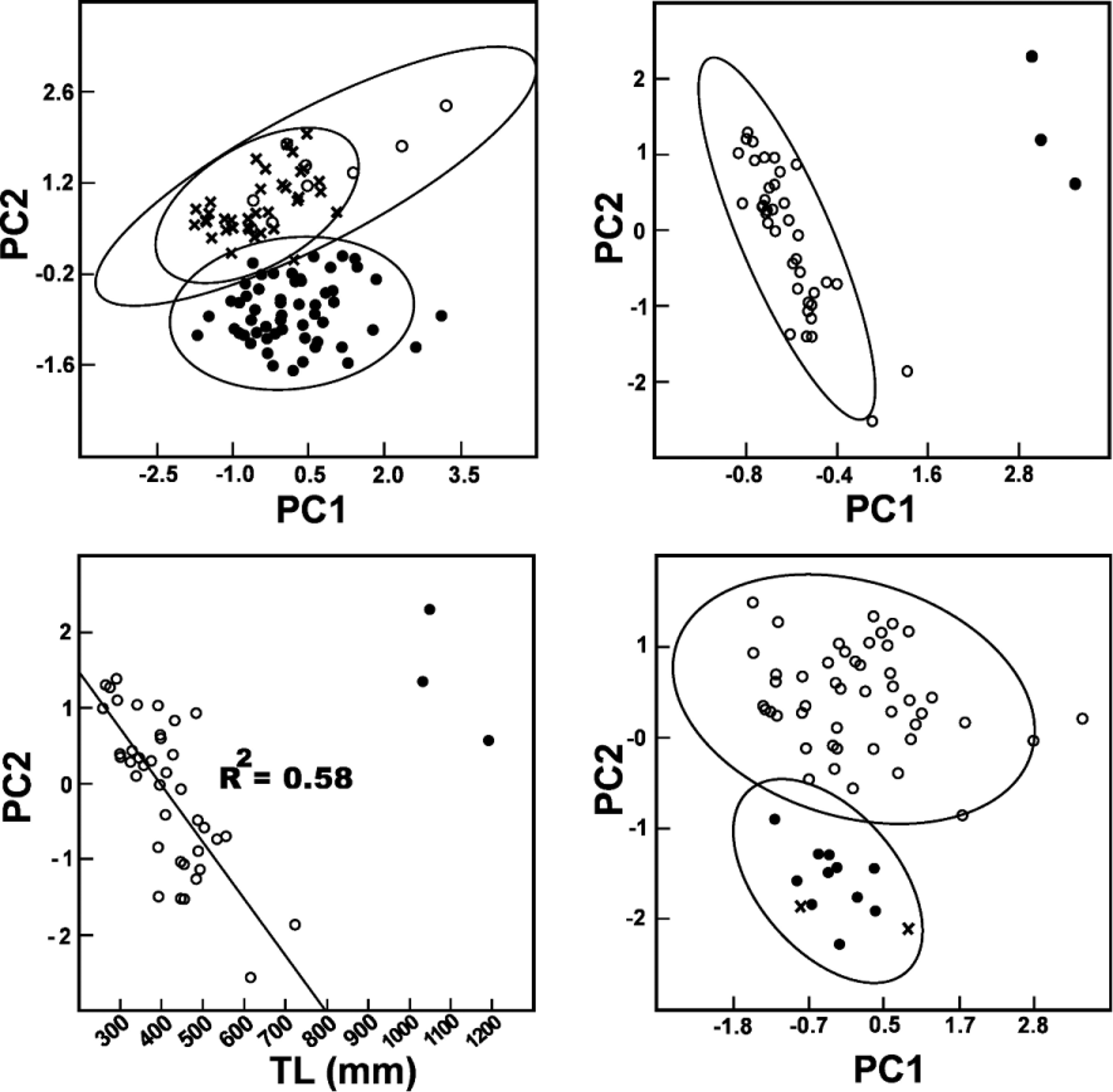
Comparisons. The comparisons and confusions in the literature involving A. snethlageae and A. major are discussed in the previous species account and will not be repeated here. With respect to Amazonian species with 17 dorsal scales, the frequent color morph of A. snethlageae is rather unique in having well-defined pale crossbands, typically edged in black, on a dark background. Some specimens of A. latifrons are somewhat similar in color pattern, but they can be easily distinguished from A. snethlageae in having one postocular (as opposed to two), a loreal that is less than twice as long as wide (as opposed to twice as long as wide), and typically having six supralabials (as opposed to typically having seven). Juvenile specimens of A. gigas have a color pattern similar to A. snethlageae but the two species differ in the number of infralabials in contact with the chinshields (three in A. gigas , four in A. snethlageae ). The less common color pattern, a pale brown background with dark blotches, is similar to A. schach (see remarks) and A. torquatus . Atractus torquatus has one postocular (as opposed to two in A. snethlageae ) without reported exceptions. Atractus snethlageae is most similar to A. flammigerus (character states in parenthesis) but differs from this species in lacking keels on dorsal scales (adults have keels on dorsal scales at the level of the vent), typically seven supralabials (typically eight), and a venter with tiny dark spots forming irregular diffuse markings (spots are solid, regular, about the size of a dorsal scale and which tend to form longitudinal stripes). According to the statistical analysis A. snethlageae and A. flammigerus also differ morphometrically ( Fig. 2 View FIGURE 2 ) with a large separation in PC2. Because SUPRAOC had the highest component loadings on PC2 we looked into this variable to see if it could be helpful for discriminating the two species. Indeed A. flammigerus has longer supraoculars than A. snethlageae and this character, for the purpose of being used as diagnostic, can be presented as SUPRAOC/HL ratio. In A. flammigerus this ratio is 0.182-0.188 whereas in A. snethlageae it is 0.093-0.152.
Color pattern. Two different color pattern types occur in this species with considerable variation within them. The most common pattern (pattern II in statistical analyses) consists of a dark brown or dark grayish brown ground coloration with cream or buff yellow dorsal crossbands that extend down to the second or third dorsal scale row on the sides. The dorsal bands typically expand longitudinally into one or two dorsal scales and are edged by black, but in some specimens the bands are irregular and form blotches that may expand longitudinally more than two dorsal scales. Rarely the black edges are inconspicuous. The crossbands may be complete across the dorsum, interrupted in the vertebral region or alternating on the sides. The interspace between crossbands is longer than the crossbands themselves, encompassing two to seven dorsal scales but with variation within individuals. The first two dorsal scales have an irregular pattern of dark and pale mottling and solid cream spots might also be present at that level. A large white or cream nuchal band is present in juveniles and small adults but gradually darkens and disappears as individuals get larger. In adults the top of the head is either the same color as the background color of the body or slightly paler. Irregular and inconspicous dark spots or mottling are frequently observed on the head. In the other, less common color pattern type (pattern III in statistical analyses) the dorsal background coloration is pale brown or creamy brown (in preservative) with a series of irregular paired dark brown blotches that may or may not contact each other middorsally. A dark brown nuchal band is usually present with the head usually darker than the dorsal background. The ventral coloration in both color pattern types is creamy with varying levels of dark brown pigmentation in the form of tiny dark spots that may form diffuse blotches. In some specimens the dark markings can form a broken midventral stripe. Rarely the venter is so heavily pigmented that it is almost uniform dark brown.
Distribution. Atractus snethlageae as currently defined is a widespread species occurring in northern Brazil, and the most part of the upper Amazon Basin in Colombia, Ecuador, Peru, Bolivia and Gran Chaco in northern Argentina (Giraudo and Scrocchi, 2000; Passos 2008). This species inhabits lowland and lower montane rainforests, from sea level up to 1800 m.
Remarks. As stated above, A. snethlageae has been repeatedly confused with A. major in Ecuador (e.g. Savage 1960; Duellman 1978; Pérez-Santos & Moreno 1991). We think that part of this confusion stems from the great amount of intraspecific color pattern variation in A. snethlageae , especially the fact that two distinct color morphs are found in this species, one of which (pattern III in this study) is similar in general aspects to the color pattern observed in A. major . Discrete polymorphism in color pattern as documented here for A. snethlageae , although rare in snakes, has been found in other species of Atractus (Fajardo 2000; Passos & Prudente 2012; Schargel, unpublished observations) and will likely become a more common observation as we obtain larger samples of poorly known species and a better understanding of the taxonomy of the genus.
The name A. badius has also been used for specimens of A. snethlageae from Argentina, Bolivia, Brazil, Ecuador and Peru in the literature or on a significant number of museum specimens that we have examined. This has been the case in the literature (e.g. Carrillo & Icochea 1995; Doan & Arizábal 2000) even after Hoogmoed (1980) had redescribed A. badius and resurrected A. flammigerus (which at the time included A. snethlageae ) from its synonymy. As a matter of fact, the name A. badius has historically been a “dumping ground” for several different species of Atractus having crossbands. Our current understanding of the species together with the examination of material and records misidentified as A. badius seems to indicate that this species is endemic to the Guiana Region as delimited by Hoogmoed (1979). Although A. badius likely occurs in southeastern Venezuela, records of this species for this country in the Coastal and the Mérida Mountain Ranges represent misidentifications of A. lancini , A. univittatus , and A. meridensis .
Comparisons between A. snethlageae and A. schach are currently problematic. The color pattern III of A. snethlageae is similar to the color pattern that has been reported in A. schach . Atractus schach was originally described from Suriname and, just like A. flammigerus , had long been considered a synonym of A. badius (see Hoogmoed 1980). The few works ( Cunha & Nascimento 1983, 1993; Martins & Oliveira 1993, 1999) that have provided taxonomic accounts for both A. schach and A. snethlageae have separated these two species solely on color pattern. As a matter of fact, before Hoogmoed (1980) resurrected A. schach , Cunha and Nascimeno (1978) had considered specimens that they examined and which they later referred to A. schach and A. flammigerus snethlageae ( Cunha and Nascimento 1983) as conspecific and under the name A. badius . At this point we have no evidence from our meristic or morphometric data that would support recognizing the two color patterns that we have assigned to A. snethlageae in western Amazonia as two different species. However, without a more comprehensive sampling we cannot establish whether what has been referred to A. snethlageae and A. schach from Brazil are conspecific or not. If specimens referred to A. schach in Brazil prove to be a different species from A. snethlageae it would still be necessary to determine whether those specimens are indeed conspecific with topotypic material of A. schach . As such, we are aware that Atractus snethlageae as herein defined might represent a species complex and a more in depth taxonomic examination of this species is in progress (Passos in prep.)
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.