Anadia blakei Schmidt, 1932
|
publication ID |
https://doi.org/ 10.1206/657.1 |
|
persistent identifier |
https://treatment.plazi.org/id/C57A878A-6615-FFB0-3BD2-0AF50A05F918 |
|
treatment provided by |
Carolina |
|
scientific name |
Anadia blakei Schmidt, 1932 |
| status |
|
Figures 12–14 View Fig View Fig View Fig
Anadia blakei Schmidt, 1932: 161–162 (holotype FMNH 17795, an adult female from ‘‘camp at altitude of 5,000 feet [1524 m] on Mount Turumiquire, [5 Cerro Turimiquire, Estado Sucre], Venezuela. Collected March 10, 1932 by E. R. Blake’’). Oftedal, 1974: 250– 252 (generic revision). Peters and Donoso-Barros, 1970: 40).
Anadia marmorata: Rivas and Oliveros, 1997: 69 (their specimen, EBRG 2746, is described below).
DESCRIPTION OF NEW SPECIMEN
The specimen (EBRG 2746) is an adult male from Cerro Humo 900 m elevation, Parque Nacional Península de Paria, Estado Sucre. Collected August 22, 1992 by Ramón Rivero (field no. RAR-1093).
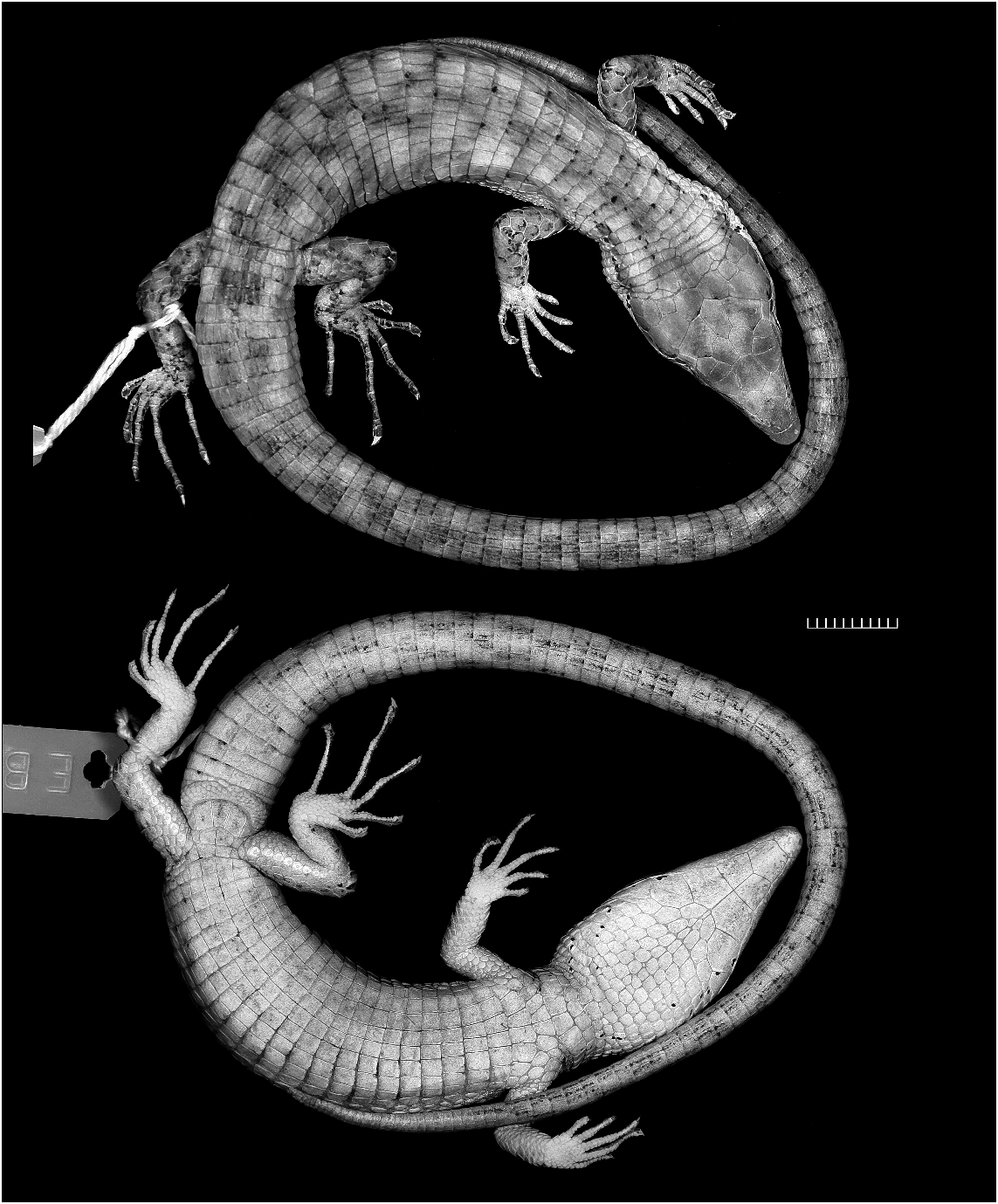
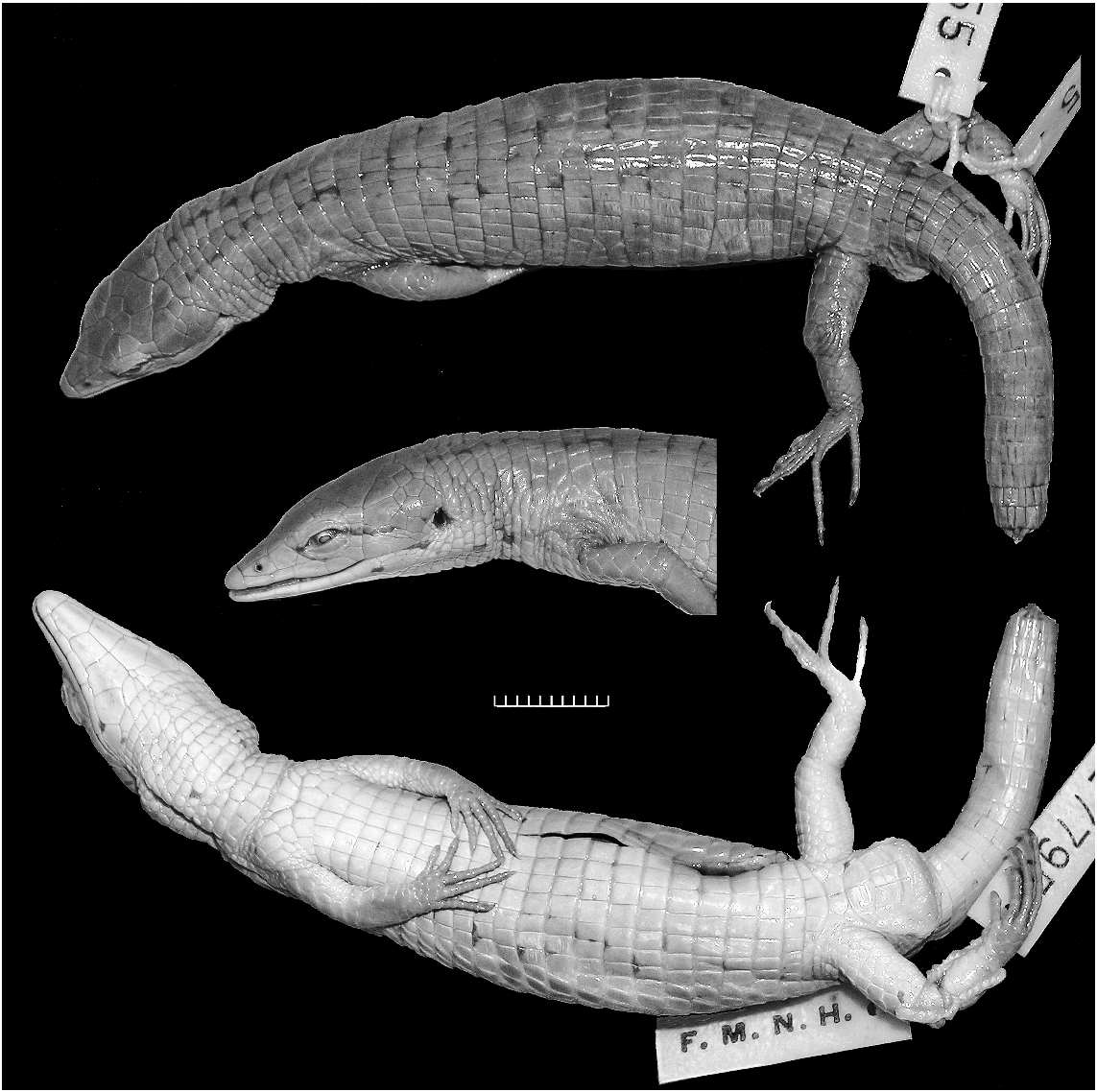
HABITUS AND PROPORTIONS: Compared with most other species of Anadia (e.g., figs. 9–11), Anadia blakei is a relatively heavy-bodied lizard. The new specimen (fig. 12) is an adult male 91 mm SVL + 157 mm tail 5 248 mm total length. The snout is attenuate and dorsally flattened, appearing nearly flat in profile. Head length 26% of SVL, 1.5 times longer than wide, 1.3 times wider than high; head swollen across temporal region (probably a secondary sex character) and distinctly wider than neck. Neck 51% of head length, 27% of trunk length. Snout-axilla length 89% of trunk length, 43% of SVL. Body wider than deep. Tail rounded. Limbs pentadactyl, all digits clawed; finger III nearly as long as finger IV, toe IV distinctly longer than toe III. Forelimb 29% of SVL, 58% of trunk length; hind leg 34% of SVL, 69% of trunk length; longest digits of appressed limbs overlap. Measurements are given in table 3.
TONGUE AND DENTITION: Tongue lanceolate, pale gray anteriorly. Upper surface of tongue behind fork covered with imbricate scalelike papillae (proximal part of tongue not examined). Anteriorly converging (chevronlike) infralingual plicae present.
Anterior maxillary and dentary teeth nearly conical in profile, becoming weakly bicuspid posteriorly (with a very small cusp on anterior face of tooth). Teeth with a slight curvature mediad and tending to be largest posteriorly, but without definite gradation in size. Large and small teeth in close proximity even anteriorly.
SCUTELLATION: Dorsum of head with normal complement of Anadia head plates ( Oftedal, 1974: fig. 1) except that frenocular is lacking in this specimen (fused with loreal). Rostral plate much wider than deep, in broad contact with nasal, laterally in contact with first supralabial, dorsally in contact with large frontonasal. Frontonasal pentagonal, with slightly convex anterior edge and pointed posterior edge. Paired prefrontals in relatively broad contact. Frontal hexagonal, widest anteriorly. Paired frontoparietals with long medial suture. Three supraoculars, anterior one largest; a tiny presupraocular on left side adjacent to first superciliary, between prefrontal and first supraocular. Interparietal hexagonal, much longer than wide. Parietals much wider than interparietal but not extending as far posteriad. Four large occipitals (postparietals) behind interparietal and parietals. Four median postoccipitals behind median pair of occipitals, larger than other dorsal neck scales.
Nasal scale entire, nostril situated in center. Nasal scale anteriorly narrowed, in broad contact with rostral anteriorly and loreal posteriorly. Loreal large, in broad contact with first superciliary and supralabials 2–3. Frenocular absent, apparently fused with loreal. One anteriorly pointed preocular and two small postoculars. First superciliary (or ‘‘presuperciliary’’) large, followed by five shorter superciliaries. No small azygous scales between superciliaries and supraoculars. Suboculars small and narrow, not forming a continuous series under eye. Eight supralabials, first seven large, the eighth small.
Upper eyelids with 10/8 ciliaries, lower eyelids with 11/12. Palpebral disk lightly pigmented, its moderately large scales not forming a median window of regular-sized panes.
Temporal scales relatively small and subequal, juxtaposed, smooth, with raised round- ed surfaces. Ear opening a vertical ovoid, edged with small pebblelike scales; tympanum recessed, transparent.
Underside of head with eight infralabials on each side (last two small). Large mental followed by larger postmental in lateral contact with first two infralabials. Three pairs of large genials in lateral contact with infralabials 2–5; first two pairs of genials in broad median contact, third pair broadly separated by median wedge of gular scales. One large postgenial on each side, in contact with last genial and in point contact with infralabial 5. Gular scales of moderate size, flat surfaced, juxtaposed. Gulars arranged in transverse rows of subequal scales, culminating in three well-defined collar rows of larger scales. Side of neck between ear and collar pebbled with subequal, rounded juxtaposed scales.
Middorsal scales 30 (table 3: note c). Dorsal scales smooth, subimbricate to imbricate, with flat surfaces. Dorsal body scales rectangular (a few irregularly shaped), longer than wide, in transverse rows only. Lateral scales similar to dorsals but smaller and somewhat variable in shape.
Ventral scales nearly square, shorter than rectangular dorsals, smooth, subimbricate, arranged in both transverse and longitudinal rows. About nine longitudinal rows of ventrals at midbody (but ventrals merge in size and shape with lateral scales, so that any count is subjective) and 23 transverse rows between collar and preanal scales.
TABLE 3 Measurements (in mm) and Scale Counts of New Specimen of Anadia blakei Schmidt, 1932
Two anterior rows of paired preanal scales. Five marginal preanal scales anterior to vent, the three middle ones larger than the lateral ones. Femoral pores weakly developed, slightly swollen pore scales in linear contact; eight pores on each thigh (including a barely discernible distal pore on left). Pores do not extend onto preanal area.
Caudal scales arranged in rows of regular width around tail, the scales disposed in transverse rows of uniformly rectangular scales on dorsal and lateral surfaces and in both transverse and longitudinal rows of larger rectangular scales ventrally. Caudal scales subimbricate, smooth.
Dorsal surfaces of upper and lower arms with large, smooth subimbricate scales; ventral sides with smaller juxtaposed scales. Hind limbs with large, smooth subimbricate to imbricate scales dorsally, the scales becoming much smaller on posterior face of thigh. Ventral side of lower leg with large, smooth, imbricate scales.
Moderate-size scales atop hands and feet. Supradigital scales single; upper and lower ungual-sheath scales covering base of claws, leaving tips well exposed. Palms and soles with small, slightly raised juxtaposed scales. No enlarged thenar scale at base of pollex. Subdigital lamellae on fingers mostly single, with a few basal ones doubled; subdigital lamellae on toes double on basal halves, single distally. Longest (4th) finger with 15 subdigital lamellae, longest (4th) toe with 20 subdigital lamellae.
COLORATION: In preservative (fig. 12) brown with grayish suffusions and indefinite black markings. See Comparisons below.
HEMIPENIS: The right retracted hemipenis of EBRG 2746 was removed for manual eversion and study especially of apical configuration; owing to misjudgment while attempting to minimize destructive dissection, the base of the tail was insufficiently opened and the organ was transversely incised an unknown distance above the base. Manual eversion was more difficult than expected owing to the stiffness of the spinulate flounces. The organ consequently was softened in a 3% solution of KOH for a total of 29.5 hours, in four sessions spaced over several days; it was stored in diluted glycerin between KOH immersions ( Myers and Cadle, 2003: 300). The following description excludes the unrecovered basal part of the organ.
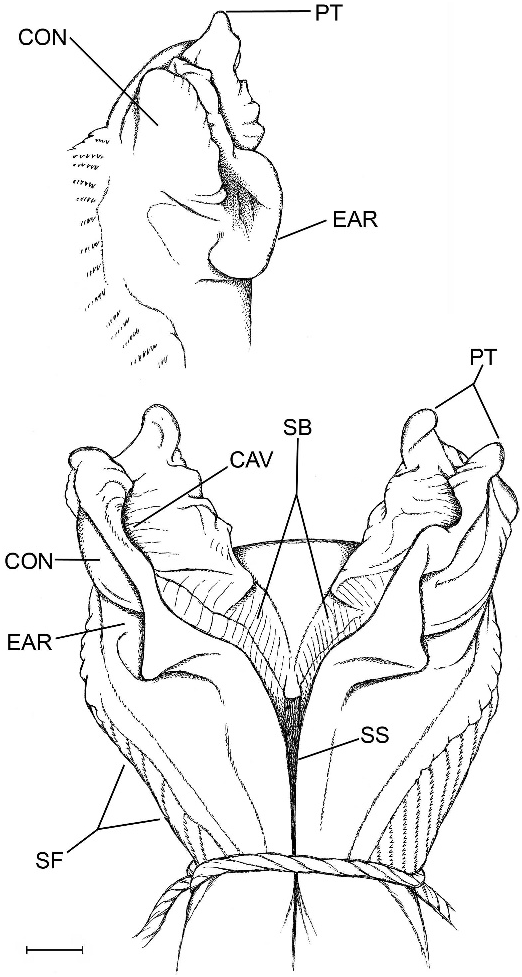
The hemipenis is distinctly bilobed with papillate tips (fig. 14). The broad medial parts of both the sulcate and distal part of the asulcate sides of the organ are nude; the nude area on the asulcate side extends into the crotch. Laterally on each side is a close-set series of proximally pointed chevron-shaped flounces bearing comblike rows of minute spinules; the branches of the chevrons are unequal, those on the asulcate side shorter. Proximally, the branches of the chevrons extend transversely across the asulcate side below the large asulcate nude area (not figured, but see ‘‘medioproximal asulcate flounces’’ defined under Notes on Hemipenial Variation in the Gymnophthalmidae ).
The sulcus spermaticus runs a medial course and bifurcates centripetally as it enters the lobular crotch; each branch then extends distally between raised walls of stiff tissue, to terminate near the end of the lobe in a concavity below a pair of large flat apical papillae. The floor of a sulcus branch is broad, especially proximally, and transversely ridged for its entire course.
In lateral view of a lobe (fig. 14, inset), the raised tissue wall that flanks the sulcus branch is convex above, which reflects the oppositeside concavity that receives the end of the branch. Proximal to this lateral convexity, the side of the raised tissue wall is concave and curved in an earlike shape. The short, distal chevrons flank the base of the lobe, but the asulcate side of the lobe is nude, continuous with the bare crotch and distal part of the asulcate side.
COMPARISONS
The male specimen from Cerro Humo compares favorably with photographs of the female holotype 11 from Cerro Turimiquire, except in having a noticeably wider head (cf. figs. 12, 13), which reflects male sexual dimorphism ( Oftedal, 1974: 250). The new specimen lacks a frenocular, agreeing with a published figure of the right side of the holotype ( Oftedal, 1974: fig. 20B); but a triangular frenocular is present on the left side of the holotype (fig. 13).
Both specimens are brown with some inconspicuous black flecking. The holotype (fig. 13) has a weak, black postocular line extending toward the ear and the hint of a pair of parallel black lines atop the neck. In the Cerro Humo specimen the black postocular line extends brokenly through the top of the ear, thence rising slightly to connect with one of the parallel lines atop the neck; additionally there is a punctuated black line extending from the mouth and across the lower edge of the ear onto the neck, giving the appearance of a black-edged pale postocular stripe ending on
11 Oftedal (1974: table 13) cited this specimen as male. However, Schmidt’s (1932: 161) original designation as ‘‘adult female’’ is supported by its relatively narrow head and nonswollen tail base (fig. 13), as compared with an adult male (fig. 12) of identical size (91 mm SVL).
the shoulder. The Cerro Humo specimen is indefinitely suffused with gray above; the underside of the head is whitish, becoming very pale brown over the venter and light brown with grayish suffusions underneath the tail.
Robust species such as Anadia blakei and A. marmorata are markedly different in habitus from the slightly built, attenuate lizards usually thought of as Anadia (compare figs. 9–11 with figs. 12–13). The monophyly of the genus remains to be established.
REMARKS
The new specimen of Anadia blakei was found by Ramón Rivero on a palm tree at 900 m on Cerro Humo. It extends the known range approximately 160 km eastward from the type locality at about 1500 m on Cerro Turimiquire. The species has also been report- ed from Cerro Negro, Caripe, roughly 60 km E of Cerro Turimiquire in Estado Monagas ( Oftedal, 1974).
Because of continuing research carried out on the Turimiquire massif and Península de Paria, it is apparent that this region has high diversity and endemism. Even though the region is still poorly known, it appears to be an area of biogeographic importance. The Turimiquire massif and the Península de Paria contain endemic elements, as well as an array of faunal and floral elements that seem to be associated with those of the central coastal range to the west, the islands of Trinidad and Tobago to the east, and the Guayana Shield and Amazonia to the south ( Steyermark, 1974; Schargel et al., 2005). Because of the key location, better knowledge of the herpetofauna of these mountains should contribute to an understanding of the evolutionary history of northern South America. Furthermore, the pressures of habitat destruction continue to impact this region dramatically. These effects are compounded by the observation that many of the known species of amphibians and reptiles are naturally highly restricted in distribution. This is particularly important for Turimiquire, which has been more impacted by varied anthropogenic activity (e.g., deforestation at all elevations, radio-communication activities, and agricul- ture in the main basin), yet receives little government protection (zona protectora Macizo del Turimiquire).
We fear that without expedient work, much of the unique herpetofauna of Turimiquire and the Peninsula de Paria will be lost before it can be properly assessed—such a loss would be highly lamentable. Anadia blakei has been included under the category of vulnerable in the third edition of the Libro Rojo de la Fauna Venezolana (‘‘Red Book of the Venezuelan Fauna’’), along with other reptiles endemic to the Turimiquire massif, such as Mabuya croizati and Atractus matthewi ( Rivas et al., 2008) .
At the moment only two species are endemic and present in the forest of both systems, namely Anadia blakei and an undescribed species of Gonatodes (Walter Schargel, personal commun.). Genera such as Riama , however, are represented by separate species in these two systems. Riama sp. is restricted to the Turimiquire summit above 2000 m (the maximum elevation on this mountain is 2400 m); R. rhodogaster occurs in cloud forest of the Península de Paria near Las Melenas on the approach to Cerro Humo, at about 700 m elevation (the maximum elevation for the peninsula is 1371 m on Cerro Humo ( Steyermark, 1973).
NOTES ON HEMIPENIAL VARIATION IN THE GYMNOPHTHALMIDAE
INTRODUCTION
Hemipenial morphology of the Gymnophthalmidae has taxonomic potential that was unexplored before the 1960s and 1970s, when Thomas Uzzell published a series of revisionary studies that included descriptions of uneverted hemipenes (especially Uzzell, 1973: 40–46). He removed, split open, and stained uneverted organs, showing that, in addition to various small spines, the hemipenes of many (most) microteiid genera have facing series of oblique or chevron-shaped flounces that bear comblike rows of calcareous spinules. The complex folding in the lobes at the tip of the uneverted hemipenis was, however, not amenable to study by dissection, and the spinule-bearing flounces on the body of organ were characterized as being in pouches or pockets. 12 After inflated hemipenes became available, the spinule-bearing flounces could be seen as protruding from the surface of the everted organ (illustrations in: Donnelly et al., 1992: fig. 3; Harris and Ayala, 1987: fig. 6; Kizirian, 1995: fig. 4; 1996: figs. 6, 10, 19, 23; Kizirian and Coloma, 1991: fig. 2; Köhler and Lehr, 2004: figs. 3, 6, 9; Myers and Donnelly, 1996: fig. 20; 2008: figs. 57, 59; Presch, 1978: figs. 1–8 13). But note that flounces lack spinules in some taxa ( Harris, 1994: figs. 5, 14, 16, 17, 20, 24; Kizirian, 1996: 141).
Harris (1994: 228) studied some retracted gymnophthalmid hemipenes ‘‘by inverting them with forceps through a lengthwise incision made in the sulcus spermaticus.’’ To carry that a step further, retracted gymnophthalmid hemipenes could be removed from preserved specimens, softened, manually everted, and inflated with petroleum jelly or other substance, following techniques used for squamates generally (e.g., see Myers and Cadle, 2003; Ziegler and Böhme, 1996). However, the spinulate flounces of gymnophthalmids such as Anadia blakei can be somewhat of an impediment to manual eversion (see page 23). Furthermore, many gymnophthalmids are very small lizards that have correspondingly tiny hemipenes, requiring care, steady hands, patience, and practice—the last not to be gained by dissection of unique specimens. Laboratory eversions of
12 The pouches or pockets noted by Uzzell presumably correspond to basinlike sections of the hemipenial wall that disappear owing to differential tissue expansion during eversion. They must not be confused with the ‘‘hemipenial pockets’’ characterizing some squamates. These retain identity in both retracted and everted states. 13 Presch’s (1978) figs. show some interesting configurations, but his drawings must be interpreted with caution. Some organs are incompletely everted, which led to serious misinterpretation in at least one case ( Myers and Donnelly, 2001: 48, footnote 21). As used by Presch, ‘‘dorsal’’ and ‘‘ventral’’ sides of everted hemipenes are equivalent to asulcate and sulcate sides, respectively. Dorsal and ventral are appropriate descriptors for retracted hemipenes of squamates, but eversion sometimes is accompanied by notable changes in the relative positioning of various structures.
Presch’s figs. 1–2 and associated description of Gymnophthalmus hemipenes deserve special note. The ‘‘elongated finger-like projections (villi)’’ and ‘‘fleshy protuberances’’ presumably are in some way homologous with the chevron-shaped folds of other gymnophthalmids, but their appearance is strikingly different.
retracted hemipenes from preserved specimens may have a smaller (less expanded) circumference than organs everted at time of death, but the terminal morphology can still be elucidated in ways not possible by dissection.
The published figures cited above were mostly of hemipenes everted at time of preservation, although in a few cases organs were softened in a solution of KOH and further inflated to test whether the apices had been fully everted. Unfortunately, collectors often fail to obtain complete eversions of gymnophthalmid hemipenes, most often perhaps because the retractor muscles are not sufficiently relaxed. The following field techniques are useful for field preparation of squamate hemipenes generally:
It is very useful and sometimes critically important for the muscles of the freshly-killed snake [or lizard] to become completely relaxed before injecting the tail base …. In order not to burst a small hemipenis nearing maximum inflation, a jeweler’s loupe may be used to monitor the everting apex—in which case it is advisable to protect one’s eyes by filling the syringe with water, which also will lessen skin exposure to formalin … The initial use of water seems not to affect the subsequent formalin-fixation of delicate hemipenial tissue and may also minimize retraction or shrinkage of the retractor muscle …. [It is] particularly important that the retractor muscles be allowed to become completely relaxed after injection of diluted sodium pentobarbital (Nembutal) or other killing agent … waiting the better part of an hour before everting hemipenes of rare [specimens] … placing specimens, especially small ones, in a jar or plastic bag with some water or damp paper towels will prevent desiccation during the wait. ( Myers and Cadle, 2003: 298)
Hemipenes everted completely (or nearly completely) in the field can later be prepared for study and illustration by techniques reviewed by Myers and Cadle (2003). Lizard and snake hemipenes occur in an extraordinary variety of shapes and sizes, and they continue to yield new morphological features of taxonomic interest. Although we speculate on the functional significance of some hemipenial structures, we recognize that we know very little about the actual shapes or degrees of expansion attained by hemipenes constrained within cloacae (the shapes of which also are subject to rarely analyzed interspecific and even intraspecific variation).
The following topics cover some aspects of hemipenial morphology that are relevant to gymnophthalmid systematics. Most character states cannot yet be adequately polarized.
COMBLIKE SPINULATE FLOUNCES
Examination of specimens and published illustrations of gymnophthalmid hemipenes shows that the comblike rows of calcareous spinules occur in varying configurations that differ between species and probably genera. One difference involves presence or absence of a distinct set of medioproximal asulcate flounces (MPAF), which are short transverse or chevron-shaped spinulate plicae proximal to the median nude space 14 on the asulcate side of the hemipenis. These plicae may connect laterally with other spinulate plicae or they may be separate, as in figure 15. They are shown as chevron shaped on retracted organs figured by Uzzell (e.g., 1973: 44, fig. 17), but shape conceivably changes to domelike or straight during eversion. In any case, the medioproximal asulcate flounces are transversely more or less straight in Anadia blakei (not illustrated in fig. 14), A. ocellata (fig. 15), and A. pamplonensis (Harris and Ayala, 1986, fig. 6).
The medioproximal asulcate flounces (MPAF) are shown on most gymnophthalmid hemipenes illustrated. They are slightly dome shaped in Cercosaura goeleti , but it is not certain that the MPAF are spinulate in this species (the other, chevron-shaped flounces of C. goeleti have minute spinules ‘‘embedded or feebly protruding at best’’ fide Myers and Donnelly, 1996: 29, fig. 20). Medioproximal asulcate flounces are lacking in some taxa, however, including Euspondylus auyanensis of this paper (fig. 6), Arthrosaura synaptolepis and A. montigena ( Donnelly et al., 1992: fig. 3;
14 The median nude space of the asulcate side of the hemipenis was termed ‘‘median welt’’ by Uzzell because of its appearance in the retracted hemipenis. It expands to smooth tissue on eversion.
Myers and Donnelly, 2008: fig. 57), and Neusticurus rudis ( Myers and Donnelly, 2008: fig. 59).
MORPHOLOGY OF BILOBED SECTION
Many (if not most) gymnophthalmids have bilobed hemipenes. Uzzell (1973: 40–41) understandably ‘‘placed little emphasis on variation’’ in the complex folding of the retracted lobes. Indeed, the apices of the lobes are often difficult to interpret even when inflated, in part because it may be impossible to determine whether the tiny lobes are completely everted (especially when one is preparing a specimen under field conditions!).
Nonetheless, the lobes and intervening crotch are a rich source of characters and are worth detailed attention, as shown by the following examples. In Arthrosaura synaptolepis the lobes are terminally flattened, with disclike apices and, in the crotch, a pronounced bumplike structure marking the division of the sulcus spermaticus ( Donnelly et al., 1992: fig. 3). In contrast, A. montigena has flounces of comblike spinulate flounces extending well onto the (fully everted?) lobes and there is a distinct, relatively deep hole (the ‘‘orificium’’) in the lobular crotch ( Myers and Donnelly, 2008: 96, fig. 57). Raised tissue at the sulcus division seems not uncommon on gymnophthalmid hemipenes, but the orificium, as interpreted, is a seemingly unique structure among lizards and snakes.
As shown in many illustrations, the everted apices of gymnophthalmid hemipenes often seem to be complexly and very irregularly folded, but this bears close consideration. If the lobes are fully inflated, some pattern may be discernible in the folding and orientation of the sulcus spermaticus (itself often broad and indistinctly bifurcated). An example is provid- ed by the new Euspondylus auyanensis , which at first glance seemed to have commonplace lobes terminating in folds resembling little hills and valleys. The sulcate face of each lobe, however, bears an easily overlooked, shallow rimmed space or ‘‘basin,’’ here interpreted as a possible concentration area for seminal fluid (figs. 6–7 and associated text).
The sulcus spermaticus normally bifurcates at the median crotch. The course of the two branches is not always obvious, although the tissue floor is usually smooth, flat, and unmarked. The sulcus branches of Anadia blakei , however, are unusually wide and bear a cross-lined pattern of minute ridges. Seminal fluid presumably flows into facing cuplike depressions near the tips of the lobes (fig. 14), very different in position from the concentration areas suggested above for Euspondylus auyanensis and below for Anadia ocellata .
NONSPINULATE ‘‘NUDE’’ HEMIPENES
The genera Alopoglossus , Ptychoglossus , Adercosaurus , and Ecpleopus have spineless hemipenes (see also Neusticurus below). The asulcate sides of the organs of the first three genera bear nonspinulate tissue ridges or flounces that are transversely aligned or dome shaped (rather than oblique and chevron shaped). In Ecpleopus , however, even the flounces are lacking ( Uzzell, 1969: 8, fig. 3).
Harris (1994: 272) observed that the hemipenis of Ptychoglossus ‘‘has [nonspinulate] flounces of the form found in macroteiids and the closely related Alopoglossus , a primitive condition among microteiids.’’ Based on this and other aspects of morphology, Harris suggested that these two genera possibly comprise a sister group to all other microteiids. Harris’ hypothesis was not cited but was nonetheless corroborated by subsequent molecular analyses. Pellegrino et al. (2001) found Alopoglossus to be the sister taxon to all other gymnophthalmids and erected the subfamily Alopoglossinae for it. Castoe et al. (2004) corrected an error in the Pellegrino et al. data set and added Ptychoglossus to the Alopoglossinae .
Castoe et al. (2004: 465) were ‘‘unable to place Adercosaurus Myers and Donnelly, 2001 , definitively in a subfamily because we were unable to examine specimens [the unique specimen had been transferred from AMNH to EBRG in Venezuela] … this genus may belong in the Alopoglossinae , the Ecpleopinae, or the Cercosaurini.’’ However, the Cercosaurini can be reasonably excluded because Adercosaurus lacks oblique or chevron-shaped spinulate flounces; the nonspinulate flounces are transversely dome shaped ( Myers and Donnelly, 2001: fig. 39).
Various genera in the classifications of Pellegrino et al. (2001) and Castoe et al. (2004) lack DNA data and are provisionally placed to subfamily based on resemblances or previous associations. We therefore feel no restraint in provisionally assigning Adercosaurus to the Alopoglossinae because of its hemipenial, lingual, and physiognomic resemblances to Ptychoglossus .
The recently described Kaieteurosaurus Kok (2005) from Guyana is another monotypic genus known from a single (female) specimen. Although differing in significant features of scutellation, Kaieteurosaurus shares with Adercosaurus and Riolama remarkable resemblances in dorsal tongue morphology (anterior and posterior plicae separated by median section of scalelike papillae) and ventral coloration (dark or dark-edged ventral scales with pale centers). We suspect that Riolama is currently misplaced in the Cercosaurini and that molecular and hemipenial data will eventually confirm that both it and Kaieteurosaurus belong with Adercosaurus in the Alopoglossinae . However, the lingual morphology of Adercosaurus , Kaieteurosaurus , and Riolama is shared by Ecpleopus (Ecpleopinae) , which also shares with Kaieteurosaurus the unusual gymnophthalmid condition of hexagonal ventral scales. Adercosaurus , Kaieteurosaurus , and Riolama are genera endemic to the western part of the Guayana ( Guiana) Shield, whereas Ecpleopus is endemic to the Brazilian Shield. It remains to be determined whether the complete lack of flounces on the nude hemipenis of Ecpleopus is primitive or derived.
COMMENTS ON NEUSTICURUS
The genus Neusticurus sensu stricto (fide Doan and Castoe, 2005: 409–411) is excluded from the above category of genera characterized by nonspinulate ‘‘nude’’ hemipenes—even though it is thought to lack calcareous spinules on the flounces. Most or all the five species (bicarinatus, medemi, racenisi, rudis , tatei) currently included in Neusticurus have very welldeveloped flounces that are similar in outward appearance to those of most other gymnophthalmids. The flounces, which are reflexed into typical chevron shapes, contain ‘‘denser supporting areas’’ (fide Uzzell, 1966: 311, for bicarinatus, rudis , and tatei). According to Uzzell, the supporting structures of the flounces (sometimes irregularly shaped) do not stain with alizarin red S.
A simple loss of calcification as an evolutionary event seems likely to have occurred if the supporting structures are homologous with calcareous spinules, which looks to be the case with Neusticurus rudis . Myers and Donnelly (2008: 99) described and illustrated the hemipenis of N. rudis as having comblike rows of ‘‘minute, presumably mineralized spinules,’’ but the tiny spinulelike structures indicated in their illustration (of the left organ from AMNH R-140208) appear noncalcareous after staining with alizarin red S in 2009 (following the method of Uzzell, 1973: 40–41).
HEMIPENIS OF ANADIA OCELLATA
The organ illustrated in figure 15 shows several hemipenial features mentioned above. The left field-everted hemipenis of AMNH R- 129779 is a tiny bilobed organ 6 mm long in sulcate view and 5 mm across. Owing to differential tissue expansion during eversion, the severed end lies not at the proximal base but higher on the sulcate side. An everted left organ from a second specimen (AMNH R- 114306) is very similar but slightly less inflated. Both specimens are from western Panama.
There are three stout, soft-tissue papillae atop each lobe; the medially positioned papilla has an elongated crested apex, paralleling the one on the opposite lobe. The sulcus spermaticus bifurcates at a ridge of raised tissue in the crotch. Each sulcus branch turns sharply laterad, running a short course up onto a broad collar flanking the sulcate sides of the apical papillae. Seminal fluid conceivably pockets in a deep depression surrounding the lateralmost papilla (suggested especially by the appearance of the less fully inflated hemipenis from AMNH R-114306).
There are several chevron-shaped flounces on the asulcate side below each lobe and, below those, two additional oblique flounces positioned more laterally. There are two transversely aligned, very pronounced medioproximal asulcate flounces that do not connect with the single lateral flounces.
The two organs discussed above are the ones mentioned by Myers and Donnelly (2001: 49) as falsifying Presch’s (1978: fig. 6) description of a bulbous, nonlobed nude hemipenis. The latter (under the synonymous name A. metallica ) appears to have been the proximal part of an incompletely everted organ.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Anadia blakei Schmidt, 1932
| Myers, Charles W., Fuenmayor, Gilson Rivas & Jadin, Robert C. 2009 |
Anadia marmorata: Rivas and Oliveros, 1997: 69
| Rivas, Gilson & Oswaldo Oliveros 1997: 69 |
Anadia blakei Schmidt, 1932: 161–162
| Oftedal, Olav T. 1974: 250 |
| Peters, James A. & Roberto Donoso-Barros 1970: 40 |
| Schmidt, Karl P. 1932: 162 |