Potamotrygon scobina Garman, 1913
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4310.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:51059A1A-74EE-4DFC-90DD-B9130CE267CB |
|
DOI |
https://doi.org/10.5281/zenodo.5618456 |
|
persistent identifier |
https://treatment.plazi.org/id/C55887A8-1751-3B29-CDB6-FE98675E658B |
|
treatment provided by |
Plazi |
|
scientific name |
Potamotrygon scobina Garman, 1913 |
| status |
|
Potamotrygon scobina Garman, 1913 View in CoL
( Figs. 2–14 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 View FIGURE 11 View FIGURE 12 View FIGURE 13 View FIGURE 14 , 32 View FIGURE 32 , 43 View FIGURE 43 ; Tables 1 –2)
Potamotrygon scobina Garman 1913 View in CoL , p. 418 (original description, rio Tocantins at Cametá, Pará, Brazil, not illustrated); Castex 1964, p. 27 (brief characterization); Castex & Maciel 1965, p. 15 (citation); Rosa 1985, pp. 324–335, figs. 22D, 79–81 (taxonomic review, redescription); Compagno & Cook 1995, pp. 72, 74, 80 (listed); Compagno 1999, pp. 495 (listed); Ross & Schäfer 2000, pp. 46, 48, 120, fig. S66080 View Materials -3 (listed); Marques 2000, p. 46 (molecular study of parasites); Carvalho et al. 2003, p. 25 (listed); Almeida 2003, pp. 1–118, appen. 4–6 (systematic and biological aspects); Bragança et al. 2004, pp. 49–59 (feeding); Charvet-Almeida et al. 2005, pp. 165–171 (reproduction); Compagno 2005, p. 541 (listed); Deynat 2006, p. 491 (citation); Magalhães et al. 2006, pp. 575–583 (venom biochemistry); Rapp Py-Daniel et al. 2007, pp. 95, 107, 122, fig. 5 (listed); Toffoli et al. 2008, p. 324 (molecular study of family); Montag et al. 2009, pp. 247, 252 (listed); Almeida et al. 2009, pp. 1–11 (distribution); Duncan et al. 2010, pp. 21, 22, 25 (gill physiology); Carvalho & Shibuya 2013, pp. 72, fig. on p. 76 (identification, rio Madeira); Fontenelle 2013, pp. 1–226, figs. 1–14, 51–55 (taxonomic review); Rosa et al. 2014 pp. 256–260 (general biology); Fontenelle et al. 2014, pp. 249, 252, 264, 266, 267 (comparison with P. limai View in CoL , described as new); Fontenelle & Carvalho 2016, pp. 253, 256, 257, fig. 5 (morphology of brain).
Paratrygon scobina: Fowler 1948 , p. 10 (listed).
[non] Potamotrygon scobina: Ferreira et al. 2011 View in CoL , p. 121 (listed); Ross & Schäfer 2000, pp. 120, 121, figs. S66091 View Materials -4, S66092- 2 (listed); Lasso et al. 2014 pp. 83, 110, fig. 79 (photos).
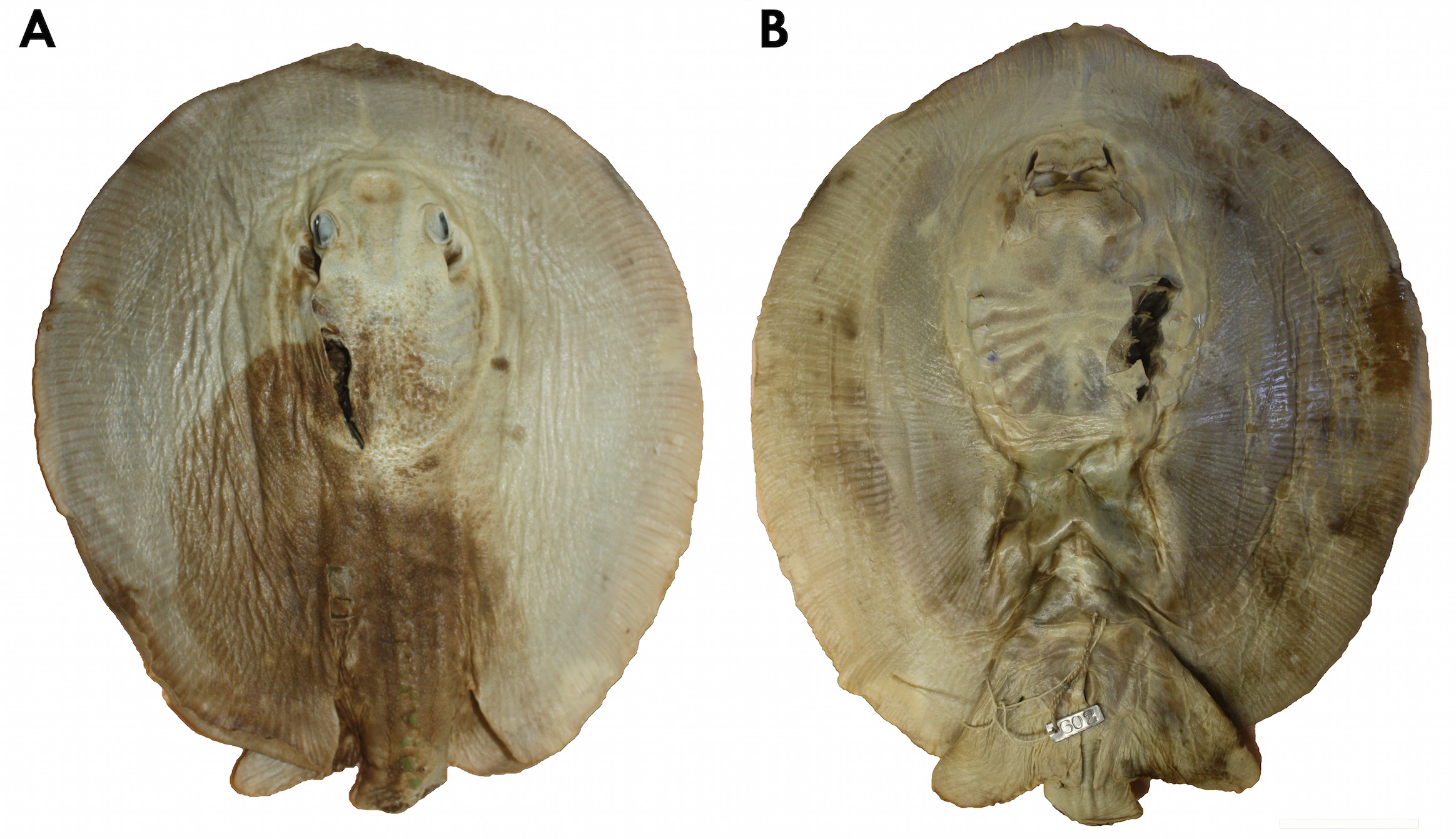
Holotype. MCZ 602 About MCZ s, juvenile male, coll. Thayer Expedition ( Figs. 2–5 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 ). Type locality. Rio Tocantins at Cametá, Pará state, Brazil.
Diagnosis. Potamotrygon scobina is distinguished from congeners by a combination of characters: disc dark brown to dark gray with relatively small beige to yellow ocelli with thin dark brownish contours; ocelli usually smaller on central disc, varying in number and size, sometimes forming rosette-like patterns or grouped around a central larger ocellus; ventral disc whitish, with darker blotches over posterolateral margins; tail dark, with nonocellated, irregular, light colored spots; labial folds absent; teeth small, with a single low cusp; dermal denticles small and relatively few in number, concentrated mostly on central disc; disc margins usually lacking denticles; rostral denticles simple, composed of a single central crown; head denticles with low, star shaped crowns; caudal denticles with same pattern as head, but smaller; one or two irregular rows of thorns on dorsal tail midline, always converging to a single row posteriorly; thorns relatively large and curved; tail long and slender, with a narrow base; cartilaginous rod notably very long and thin; a third, smaller, lateral angular cartilage present. Potamotrygon scobina is distinguished from congeners by its color pattern (further detailed below) and in having a third, smaller, lateral angular cartilage, except P. limai , P. adamastor sp. nov., P. garmani sp. nov., and P. amazona sp. nov. From P. limai , P. scobina is further distinguished by lacking its polygonal color pattern on disc, and by having a thinner tail and dermal denticles with lower crowns and fewer dichotomies. From P. amazona sp. nov. and P. adamastor sp. nov., by having a less muscular disc in adults, comparatively smaller and fewer dermal denticles, larger and better defined ocelli on disc, and a more slender and longer tail (mean tail width 13.4% DW vs. 19.0% DW in P. adamastor sp. nov. and 16.7% DW in P. amazona sp. nov.; mean tail length 121.5% DW vs. 78.1% DW in P. adamastor sp. nov. and 89.3% DW in P. amazona sp. nov.). From P. garmani sp. nov., P. scobina is differentiated by presenting a darker disc background, smaller ocellated spots, a longer and more slender tail (mean length 121.5% DW vs. 100.6% DW in P. garmani sp. nov.; mean width 13.4% DW vs. 14.1% DW in P. garmani sp. nov.), more tooth rows in both jaws (44/48–50 vs. 32–40/ 33–40 in P. garmani sp. nov.), and in having comparatively smaller thorns in dorsal mid tail series.
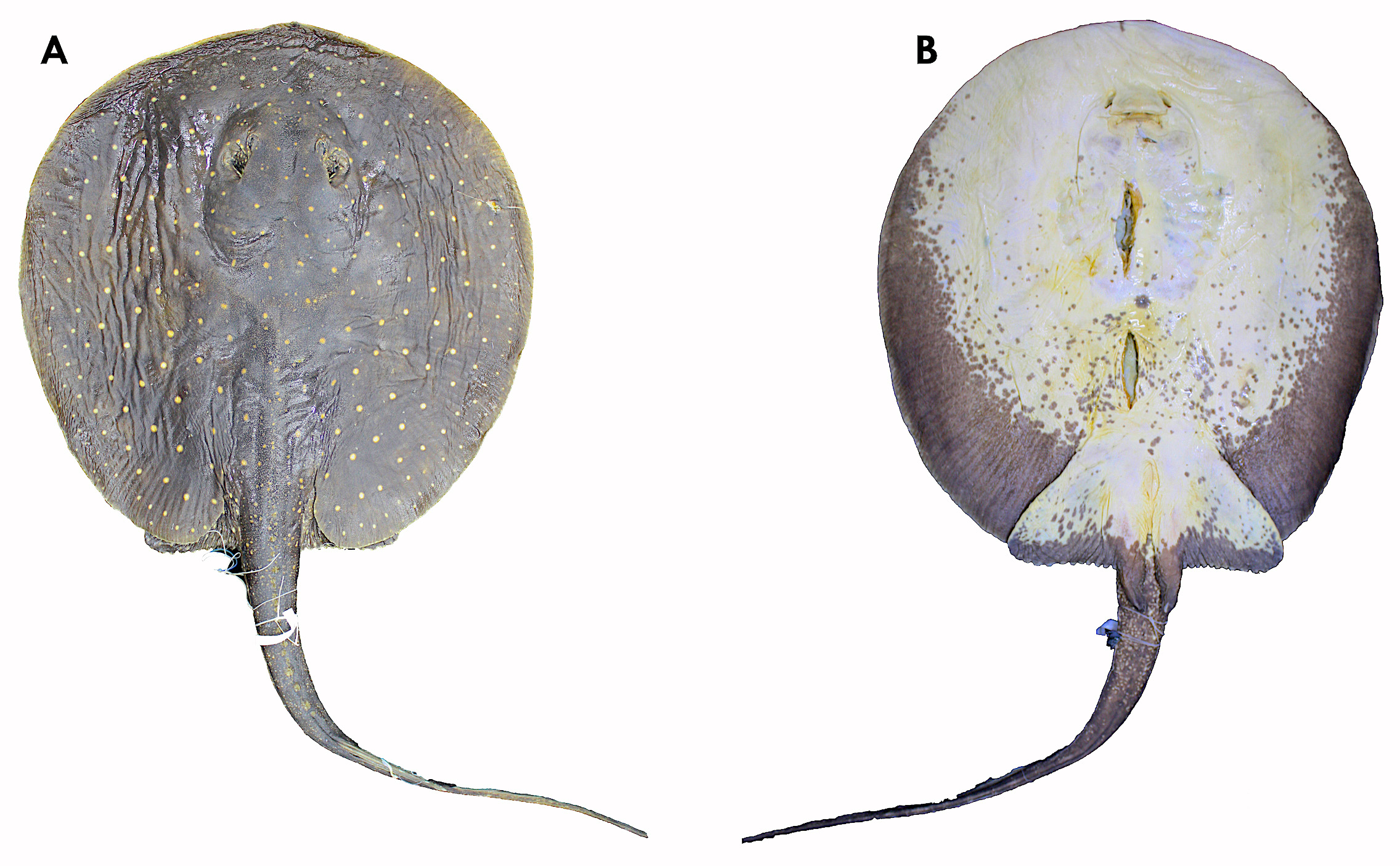
Description. Disc oval, slightly longer than wide (DL 98.8–112.4% DW) ( Figs. 2 View FIGURE 2 , 6 View FIGURE 6 ). Rostral portion of disc broadly oval, presenting a small round protuberance on anterior snout ( Figs. 3 View FIGURE 3 a, 7a). Disc dorsoventrally slender and comparatively thin at margins. Eyes small and oval (mean 3.0% DW), around two times smaller than spiracles; spiracles rhomboidal, obliquely set just posterior to eyes ( Figs. 3 View FIGURE 3 a, 7a). Head flat, low, not abruptly or significantly raised above disc, head length about 1/3 of disc length, with interorbital distance 12.9–17.5% DW, and interspiracular distance 15.9–21.5% DW. Nasal curtain extending to mouth opening. Mouth small and lightly undulated or convex, with a central notch; mouth width 7.1–12.5% DW, and internasal distance 7.3–9.2% DW ( Figs. 3 View FIGURE 3 b, 7b). No labial folds or ridges present. Five buccal papillae present on mouth floor, two posterior and three anterior; three papillae in a single row on mouth roof. Branchial basket wider than long, with distance between first branchial slits 23.1–29.0% DW, and distance between fifth branchial slits 15.9–21.2% DW.
Teeth small, simple and rounded, wider than long, set in quincunx on narrow and arched upper tooth plate, and wide and trapezoidal lower tooth plate ( Figs. 4 View FIGURE 4 a–d, 8). Tooth row count 40 in upper jaw and 48–50 in lower jaw. Adult males presenting a single, central pointed cusp on midrow teeth in both jaws. Lateral teeth in adult males, juvenile males, and females simple, with a single rounded cusp. All teeth with a single cutting surface. Lower jaws considerably wider than upper jaws, always with more tooth rows.
Pelvic fins broad, subtriangular, their length ranging from 23.7–60.3% DW (mean 52.8% DW), with rounded apices and undulated posterior margins ( Figs. 3 View FIGURE 3 d, 7d). Pelvic fin posterior margins project slightly beyond posterior disc. Anterior pelvic margin length varying from 20.5–27.8% DW. Claspers robust, long and dorsoventrally compressed ( Fig. 7 View FIGURE 7 d). Clasper base more robust and wider than clasper tip. Clasper external length 3.9–14.2% DW, and internal length 7.9–21.5% DW (considering juveniles and adults). Clasper groove curved medially, with a semicircular apopyle, and an oval and large hypopyle. Dorsal pseudosiphon oval, obliquely positioned. Ventral pseudosiphon well developed, elliptical and medial.
Tail slender, long and well developed ( Figs. 6 View FIGURE 6 , 7 View FIGURE 7 c). Tail width ranging from 10.1–20.2% DW (mean 13.4% DW), and length ranging from 76.3–145.2% DW (mean 121.6% DW). Tail narrows only slightly from base to caudal sting origin, then tapers posteriorly more intensely toward extremity. Long and slender cartilaginous rod present internally in tail more or less as of caudal sting, its length generally 1/2–2/3 of total tail length. A single irregular dorsal row of tail thorns present; thorns with a wide and round basal plate and posteriorly curved, only moderately developed crown; sometimes two irregular rows present on tail base. Tail also with small and scattered denticles. Small, single lateral row of spine on tail, originating at caudal sting origin. Caudal sting length 13.8– 25.8% DW.
Coloration. Dorsal disc dark brown to dark gray ( Figs. 3 View FIGURE 3 c, 9), with small white to yellow irregular spots, varying in number. Beige to yellowish ocelli present, their outer halo thin, darker, and poorly defined; ocelli same size or smaller than eyes, but usually larger between disc margins and center; ocelli spread over all of disc. Smaller irregular whitish spots sometimes present, set around a central, larger ocellated spot. Four main color patterns observed, but intermediate specimens also exist: (i) disc dark brown, with relatively large ocelli varying in quantity, and without accessory smaller spots; (ii) disc dark brown, with large ocellated spots, and with accessory small and irregular whitish spots mainly on posterior dorsal portion of disc; (iii) disc dark brown, with few, small ocelli, and sometimes very small accessory spots; (iv) disc dark background with numerous irregular whitish spots, sometimes organized around ocelli; ocelli sometimes discernible only on disc margins, but present on tail base and dorsal pelvic fins. Ventral disc white to creamy-white, sometimes with central darker spot. Subadults and adults with ventral posterolateral dark gray blotches on disc, extending anteriorly to level of pectoral girdle. Ventral darker pigmentation age related; younger specimens generally lighter.
Dorsal pelvic fins with same pattern as disc margins, with a dark background and ocellated spots. Posterior pelvic margin with lighter color. Ventrally, pelvic fins light with a posterior dark margin, varying with specimen size and age. Claspers dorsally with same background pattern as pelvic fins, but with irregular clear spots, without ocelli. Ventrally, claspers dark. Dorsal tail dark, similar to dorsal disc color, covered by small irregular, whitish spots. Lateral tail with small, spaced groupings of very small whitish irregular spots. Ventrally, tail whitish, covered by dark blotches, continuous with those of disc. Dark blotches progressively smaller from anterior to posterior tail. Adults may present a more uniform dark ventral tail.
Newborn specimens may present proportionally larger marginal ocelli combined with a central roughly hexagonal pattern of smaller spots on disc (newborn frequently misidentified as P. motoro or P. orbignyi ).
Dermal denticles. Dermal denticles small and comparatively few, present on central snout, head, posterocentral disc, and dorsal and lateral tail ( Figs. 4 View FIGURE 4 e–h, 10, 11). Disc margins smooth, denticles either absent or very small and sparse. Simple denticles present between eyes in a semicircular pattern, with a single, low pointed crown. Denticles usually uniform in size, but larger denticles reach up to twice size of smaller denticles. Denticle basal plate (Bp) rounded and low. Denticles on head and central posterior disc regions star shaped, with a posteriorly set, well-developed coronal plate (Cp). Anterior end of coronal plate wider and obliquely set. Two anterior coronal ridges (Cr) present with two different levels, marked by a central rounded notch, and converging to anterior end of coronal plate. Two posterior coronal ridges present, straight and opposite, laterally set to posterior end of coronal plate. Irregular coronal dichotomies (Cd) sometimes present on coronal ridges. Denticles of caudal region with well-developed coronal plates, curved posteriorly, with two anterior and two lateral posterior coronal ridges. Basal plate fairly prominent on disc denticles. Caudal denticles more prominent, simpler and with more slender crowns than denticles on central disc. One irregular row of enlarged thorns over most of tail, with one to two irregular thorn rows over tail base. Enlarged thorns moderately robust, presenting a single posteriorly set crown and semi-oval basal plate. Lateral caudal spine rows sometimes present, posterior to origin of caudal sting, and composed of minute denticles with single crowns.
Ventral lateral-line canals. ( Fig. 12 View FIGURE 12 ). Hyomandibular canal (HYC) projecting anteriorly from the nostril and obliquely towards disc margins, curving posteriorly following anterolateral disc margin. Anterior subpleural tubules (AST) short and straight. Subpleural component of the hyomandibular canal (SPC) slightly undulated, projecting posteromedially. Hyomandibular canal abruptly curves inward by anterior margin of pelvic fin, forming the subpleural loop (SPL). Posterior subpleural tubules (PST) absent. Jugular component (JCH) projects anteriorly and obliquely, bordering external margin of branchial basket. Jugular canal (JUG) curved externally, extending anteriorly to the small loop formed by the supraorbital canal (SOC). From the posterior jugular loop (PJL), the posterior portion of the infraorbital canal (IOC) projects posteroexternally forming the external margin of the short posterior jugular loop (PJL). Infraorbital canal extends anteriorly, parallel to the anterior expansion of the hyomandibular canal. At its most anterior segment, the infraorbital canal curves medially to delimit the anteriorly positioned suborbital loop (SOL). Nasal canal (NAS) projects anteriorly, curving medially as it reaches nostril. The orbitonasal component (CON) of the supraorbital canal (SOC) extends anteromedially from the confluence between the jugular and nasal canals, forming the lateral margin of the anterior jugular loop (AJL). The orbitonasal component extends toward the ventral snout area, presenting undulations varying in size, and forms the lateral margin of the acutely shaped prenasal loop (PNL).
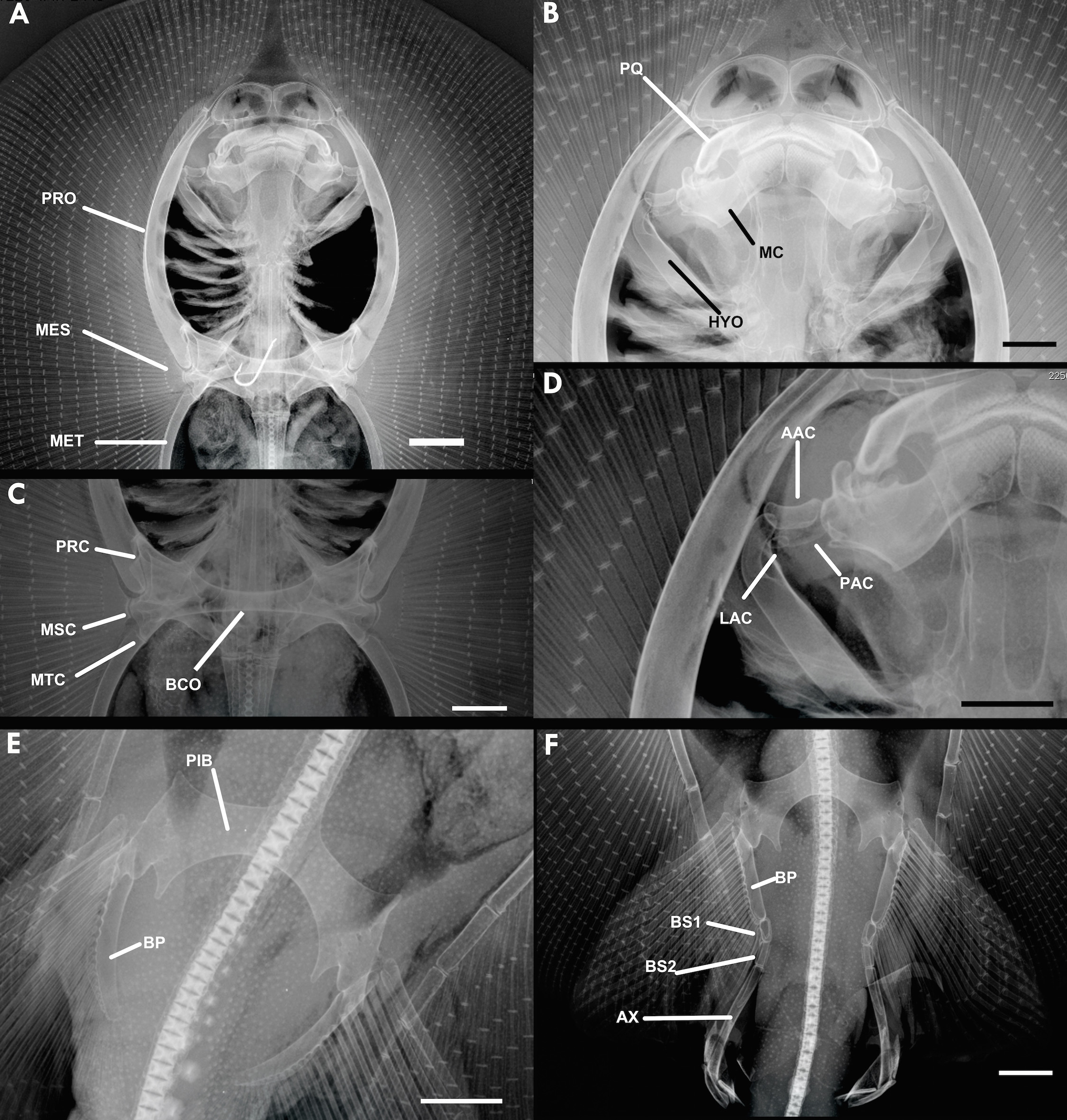
Skeletal morphology. Neurocranium. Nasal capsules ventrolaterally expanded (NC) ( Figs. 5 View FIGURE 5 , 13 View FIGURE 13 ), their anterior margin oval and convex. Precerebral fontanelle (PCF) wide and subcircular, with straight anterior margins, posteriorly delimited by a subtriangular epiphysial bar (EBP). Frontoparietal fontanelle (FPF) coneshaped, progressively narrowing posteriorly, presenting a slight median constriction, with a round posterior margin at posterior level of postorbital processes. Together, both fontanellae keyhole-shaped. Supraorbital processes (SOP) small, subtriangular, laterally projected. Postorbital process (POP) prominent, long and wide, diagonally projected anteriorly, extending to level of posterior angular cartilage. Prespiracular cartilage (psc) curved, forming a concave anterior wall to the spiracle.
Jaws and hyomandibular arch. Hyomandibular cartilage (HYO) elongated and anterolaterally projecting from neurocranium, bearing a slight lateral constriction close to posterior extremity; anterior margin of hyomandibulae curved ( Figs. 5 View FIGURE 5 b, d, 13b, d, 42). Three angular cartilages present. Anterior angular cartilage (AAC) concave, about 1/4 length of hyomandibulae. Posterior angular cartilage (PAC) oval, around same size of anterior angular cartilage, but more slender than anterior angular cartilage. Lateral angular cartilage (LAC) subcircular and small, set between posterior angular cartilage and hyomandibulae, around 1/3 length of posterior angular cartilage. Meckel's cartilage (MC) robust, its internal margin with subrectangular corners, and rounded external margins. Well defined, central concavity present on anterior margin of Meckel's cartilage. Posterior margin bearing a prominent, anterior ventrolateral process (VLP), not contacting angular cartilages, and a robust anterolateral process (LAP). Palatoquadrate (PQ) smaller and more slender than Meckel's cartilage, with a posterior concavity, laterally limited by a small triangular projection. Posterior triangular projection (PTP) well developed. Small ligament present between palatoquadrate antimeres.
Synarcual cartilage. Anterior synarcual articulates with neurocranium through a rounded, central odontoid process ( Fig. 5 View FIGURE 5 b). Medial crest extending over almost entire synarcual length, but not reaching its anterior extremity. Articular surfaces for scapular processes laterally projected, separated by concavity on each side. Anterior articular surface slightly rectangular and more prominent then rounded and lateroposteriorly projected posterior articular surface.
Pectoral girdle. Pectoral girdle articulates with synarcual through robust scapular processes ( Figs. 5 View FIGURE 5 a, 13c). Anterior process slender and straight, projected diagonally; posterior process more robust, with a small rounded extremity delimiting an adjacent concavity. Scapular process presents a biconcave medial bar, with considerably expanded lateral portions. Procondyle ( PRC) anteriorly positioned, rounded. Mesocondyle (MSC) rounded, anterolaterally positioned; metacondyle (MTC) rounded, but less so, posteriorly positioned. Robust and elongated propterygium (PRO) articulated anteriorly to procondyle and scapular girdle. Mesopterygium (MES) small, its anterior margin more developed, articulating to shoulder girdle near mesocondyle. Metapterigium (MET) posteriorly set and elongated, articulates at level of metacondyle (MTC), and curved, projecting posteriorly.
Pelvic girdle. Prepelvic process (PPP) elongated, anteriorly projected, bordered by anterior concavities of pubosquiadic bar (PIB); puboischiadic bar with expanded extremities ( Figs. 5 View FIGURE 5 c, 13e, f). Evident subtriangular lateral prepelvic processes (LPP) present. Iliac processes (IP) laterally and posteriorly set and robust. A slender and subtriangular isquial process (ISP) set more medially, projecting posteriorly. Between both isquial processes, four well developed obturator foramina (OF) present on each side.
Clasper skeleton. Basal segment 1 (B1) with a wider anterior margin ( Figs. 13 View FIGURE 13 f, 14); both margins oval. Basal segment 2 (B2) cylindrical, with a central concavity on its external wall. Both basal segments about equal in length, but segment 2 more slender. Axial cartilage (AX) elongated and cylindrical, robust, curved externally, S-shaped posteriorly. Ventral marginal cartilage (VM) diamond-shaped, projecting laterally and ventrally. Beta cartilage (BE) slender and cylindrical, about 1/2 of total clasper length, and externally curved, articulating with basal segment 1. Dorsal marginal cartilage (DM) well developed and oval, about 1/ 4 in total clasper length, curved internally. Dorsal terminal 2 (DT2) oval, slightly slender and curved, presenting a small concavity on its anterior margin. Accessory terminal cartilage (AT) straight, more robust than dorsal terminal 2, dorsal marginal and ventral marginal cartilages, with a curved anterior margin. Ventral terminal cartilage (VT) well developed, about 1/3 of clasper length, with a dilated posterior portion but rest of structure dorsally curved, almost completely covering accessory terminal cartilage.
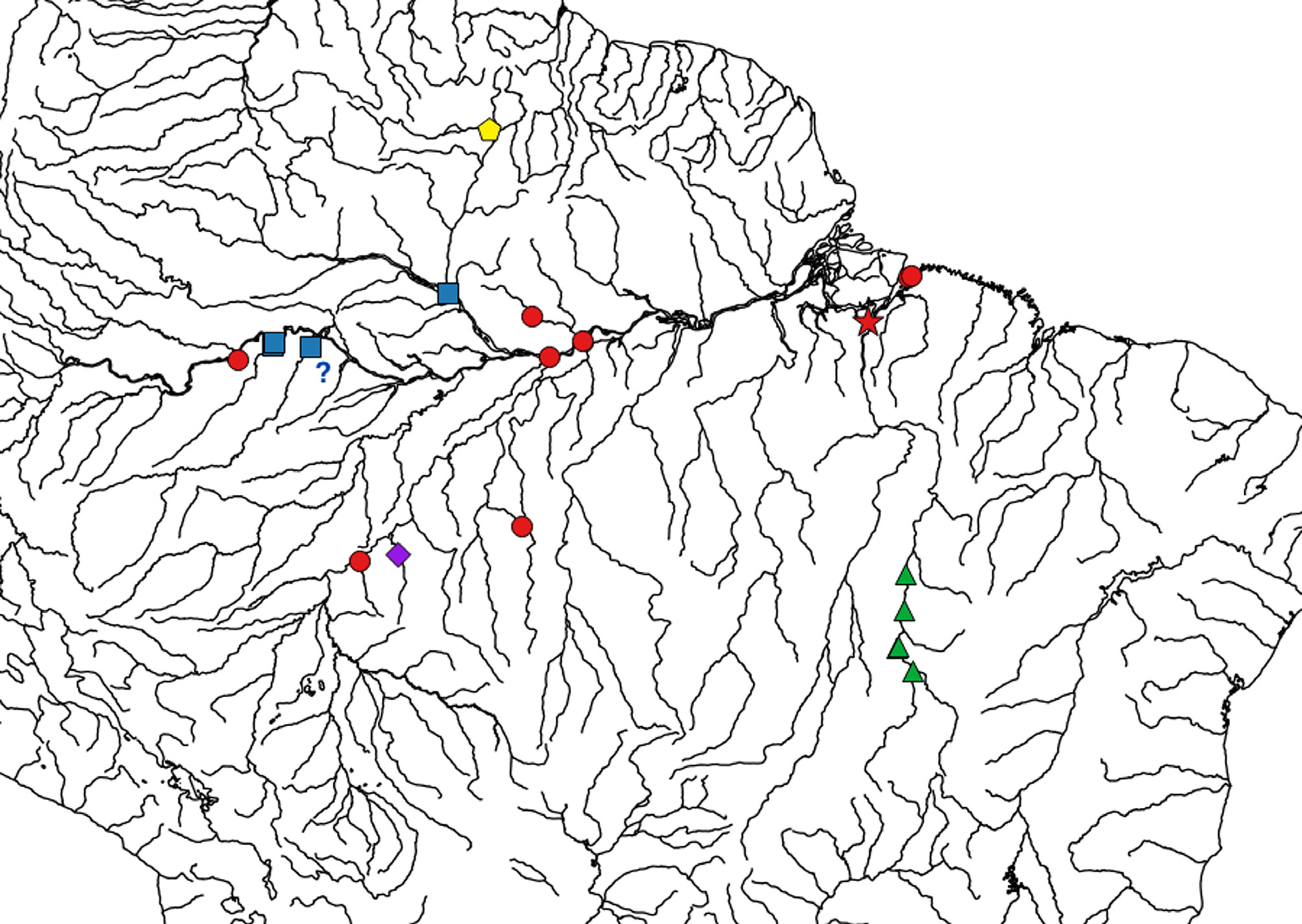
Geographic distribution. Potamotrygon scobina is widely distributed in the Amazon basin ( Fig. 43 View FIGURE 43 ). Examined specimens are from rio Tocantins (including specimens from very close to type locality) and rio Madeira systems, as well as from rios Amazonas and Solimões. There are also records from río Orinoco in Colombia and Venezuela (Rosa et al. 2014; also possibly Ross & Schäfer 2000), and scobina -like specimens occur as far as rio Nanay, in Peru (M. Kolmann, pers. comm); many of these require further checking as we were not able to examine material from countries other than Brazil.
Remarks. Although not greatly detailed in the diagnosis above, P. scobina can be distinguished from congeners by its dorsal color pattern (for more information on species listed below, see Silva & Carvalho 2011, 2015, Loboda & Carvalho 2013, Fontenelle et al. 2014, Carvalho et al. 2016, and Carvalho 2016a – c). From P. motoro , P. ocellata and P. boesemani , P. scobina is distinct in lacking red to orange contours in ocelli, which are usually smaller in P. scobina . From P. signata , P. magdalenae , P. pantanensis , P. amandae , P. falkneri , P. histrix , P. schuhmacheri , and P. tatianae by lacking complex patterns of vermiculate, oval or reniform spots. From P. brachyura , P. orbignyi , P. marinae , P. constellata and P. humerosa , P. scobina is distinguished by not having a complete or incomplete reticulated dorsal color pattern. From P. albimaculata , P. rex , P. leopoldi and P. henlei by lacking bright yellow to orange dorsal spots and/or ocelli over a blackish-brown background; from P. schroederi and P. tigrina by not having a vivid yellow and black vermiculate pattern; from P. jabuti by lacking its conspicuous ocelli and complex pattern of vermiculate markings; from P. yepezi by not having a gray dorsal color with small, irregular black spots; and from P. wallacei by not having its distinct amphora-shaped mark on dorsal mid-disc. In terms of color pattern, P. scobina is most similar to P. limai , P. adamastor sp. nov., P. garmani sp. nov., and P. amazona sp. nov. (see Diagnosis above for their separation).
Garman (1913) did not designate a holotype for P. scobina in his original description. However, he described this species based on a single juvenile male. Sex, maturity, size, measurements and label and registration information of specimen MCZ 602s are compatible with Garman's description, and therefore it is the holotype, as previously found by Rosa (1985) and Carvalho et al. (2003). The holotype is in acceptable condition, presenting a practically intact disc but with some lacerations, such as the removal of a dorsal skin fragment near the neurocranium, dissection of the jaws, and the lack of most of the tail. Its coloration is extremely faded, aside from few regions on the left disc where the original color pattern can be verified. In general, this specimen provides a fair amount of informative data, but the long and slender tail, with an elongated cartilaginous rod, a fundamental character to identify this species, is lacking in the holotype. Garman (1913) does not write about the tail in his original description probably because it was already missing; he also does not mention dorsal disc denticles, only the thorns of the dorsal tail rows (two irregular rows anteriorly, merging into a single irregular row posteriorly, composed of moderately developed hook-shaped thorns with a broad base). Rosa (1985) refers to these rows as parallel and irregular, and composed of low thorns (in relation to their basal diameter); Rosa also refers to the denticles on dorsal disc, these being small and more developed on central disc. Regarding disc dimensions, Garman only presents the length and width of the specimen (9.75 inches [= 247.6 mm] in length by 9.4 inches [= 238.7 mm] in width), while Rosa presents proportions between these dimensions (disc length ranging from 1.03 to 1.1 times disc width). There are no original drawings of the holotype.
Garman's (1913) original description of this species, based solely on the holotype (a juvenile male lacking the tail), was based on the following characters: disc round, with a small protuberance on anterior snout; mouth small, its width 1/3 distance between snout and mouth, mouth opening slightly undulated, oral epithelium with 5 internal papillae in two rows, anterior row with a bifid central papilla, posterior row bearing two papillae; teeth small, with transversally concave, smooth cusp; eyes small; spiracles slightly larger than eyes; interspiracular distance contained in 1.5 times distance between eyes and anterior snout; dorsal disc and tail covered with small and closely set denticles, with star-shaped bases; denticles greater on head, central disc region and anterior tail; enlarged dorsal tail thorns anteriorly in two irregular rows; thorns low, hook-shaped, with wide bases, and extending posteriorly on tail; ventral portion of disc smooth; dorsal disc brownish, with few sparse whitish spots; ventral disc lateroposteriorly brownish, with small white spots and brown blotches. These characters described by Garman (1913) are consistent with specimens from the lower Amazon and lower Tocantins rivers, and some regions of rio Madeira, but also apply to specimens that represent distinct species similar to P. scobina discovered in the present study (described below).
Rosa's (1985) study was the first to provide clear, unequivocal diagnostic characters for this species: subcircular disc (oval not round), with dorsal color pattern between light and dark brown bearing small yellow to white spots; teeth presenting flat crowns, wider than long; tail dorsal thorns relatively low in relation to the diameter of their bases, set in irregular parallel rows. Rosa (1985) also correctly indicated that Castex (1967) misidentified the holotype of P. scobina as a specimen of P. motoro , and presents diagnostic characters for both species ( P. scobina has smaller teeth, less sharp and smaller thorns on dorsal tail, and lacks large ocellated spots on dorsal disc). We have increased this distinction to include differences in tail length, as the tail in P. scobina is considerably longer, the lack of red to orange contours in ocelli, and by presenting a comparatively more dorsoventrally slender disc. Rosa (1985) and Garman (1913) also describe P. scobina as bearing light colored spots on dorsal disc, a character that is indeed consistent in this species, but we note that the presence of darker halos forming ocelli is common.
Rosa's (1985) measurements and counts for P. scobina are generally within the ranges reported in the present study (for details, see Rosa 1985: table 21, p. 327). For tail length, as Rosa did not have access to specimens with intact tails, his reported values are expectedly smaller than the ones presented here. Regarding counts, there are overlapping ranges between Rosa's and our data, even though Rosa's data are from a much smaller number of specimens. It is important to note that some of our counts overlap with Rosa’s at the higher end of the range; for example, the number of caudal vertebrae (101–108 in this study, 97–101 in Rosa, 1985). This is probably a function of the different number of analyzed specimens. Our data concerning number of tooth rows is the only count that does not match Rosa’s or Garman’s accounts. We found 44/48–50 tooth rows, while Rosa presents 30– 36/32–40 and Garman 34/25. This discrepancy is probably due the size of the specimens examined, as we examined many large specimens not available for their studies.
Additional material. (53 specimens). MCZ 602 About MCZ s (holotype, juvenile male, 238 mm DW), rio Tocantins , Cametá, Pará, Brazil ; UFRO-I 0 0 0 515 (juvenile male, 310 mm DW), Foz do igarapé Karipuna , rio Madeira, Jaciparaná, Rondônia, Brazil, 9°17’4.29”S, 64°23’56.67”W GoogleMaps ; INPA uncat. field no. E3 188773 (male), same data as UFRO-I 000515 GoogleMaps ; INPA 1774 View Materials (adult male, 330 mm DW), rio Uatumã (Balbina), Presidente Figueiredo, Amazonas, Brazil ; INPA uncat., field no. Ari 66 (juvenile male, 278 mm DW), rio Aripuanã, Amazonas, Brazil ; INPA uncat. (juvenile? female, 206 mm DW), same data as INPA “ Ari 66” ; INPA 29507 View Materials (adult female, 620 mm DW), same data as INPA “ Ari 66” ; INPA 15113 View Materials (juvenile male, 183 mm DW), Careiro perto da Balsa, rio Solimões, Amazonas, Brazil ; INPA 9096 View Materials (juvenile male, 298 mm DW), Janauacá , rio Solimões, Amazonas, Brazil, 3°17’41.57” S 60°20’31.34” W GoogleMaps ; INPA 9104 View Materials (adult male, 547 mm DW), same data as INPA 9096 View Materials GoogleMaps ; INPA 10395 View Materials (adult male, 328 mm DW), same data as INPA 9096 View Materials GoogleMaps ; MPEG uncat., field no. 910 (juvenile male, 347 mm DW), Ilha de Colares , rio Tocantins, Pará, Brazil ; MPEG uncat., field no. 903 (subadult male, 321 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 888 (79) (female, 365 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 38 (adult male, 520 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 63 (adult female, 595 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 0 5 (juvenile male, 516 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 19 (juvenile male, 305 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 39 (adult male, 386 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 0 29 (female, 381 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 392 (adult male, 416 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 390 (female, 372 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 385 (adult male, 364 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 494 (female, 326 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 496 (subadult male, 356 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 282 (adult male, 365 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 583 (adult female, 389 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 0 53 (adult female, 611 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 729 (adult male, 408 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 843 (adult male, 403 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 726 (adult female, 406 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 744 (adult male, 341 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 727 (female, 316 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 742 (adult female, 660 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 747 (adult male, 470 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 2F 2/2 0 4 832 (adult male, 392 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 837 12 (subadult male, 368 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 746 (subadult male, 328 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 738 (subadult male, 347 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 741 (adult female, 433 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 740 (adult female, 378 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 748 (adult male, 464 mm DW), same data as MPEG uncat., field no. 910 ; MPEG uncat., field no. 786 (adult female, 411 mm DW), same data as MPEG uncat., field no. 910 ; MZUSP 104247 View Materials (adult male, 503 mm DW), Baia de Marajó , Colares, rio Tocantins, Pará, Brazil, 0°55’34.68”S, 48°17’25.44”W GoogleMaps ; MZUSP 104245 View Materials (adult male, 543 mm DW), same data as MZUSP 104247 View Materials GoogleMaps ; MZUSP 104244 View Materials (adult male, 378 mm DW), same data as MZUSP 104247 View Materials GoogleMaps ; MZUSP 104256 View Materials (adult male, 414 mm DW), same data as MZUSP 104247 View Materials GoogleMaps ; MZUSP 104261 View Materials (female, 314 mm DW), same data as MZUSP 104247 View Materials GoogleMaps ; MZUSP 104243 View Materials (adult female, 459 mm DW), same data as MZUSP 104247 View Materials GoogleMaps ; MZUSP 104258 View Materials (adult female, 405 mm DW), same data as MZUSP 104247 View Materials GoogleMaps ; MZUSP 104259 View Materials (adult female, 298 mm DW), same data as MZUSP 104247 View Materials GoogleMaps ; MZUSP 104266 View Materials (adult male, 392 mm DW), same data as MZUSP 104247 View Materials GoogleMaps ; MZUSP 104262 View Materials (adult male, 426 mm DW), same data as MZUSP 104247 View Materials GoogleMaps ; MZUSP 104257 View Materials (adult male, 429 mm DW), same data as MZUSP 104247 View Materials GoogleMaps ; MZUSP 104268 View Materials (adult female, 396 mm DW), same data as MZUSP 104247 View Materials GoogleMaps ; MZUSP 104246 View Materials (adult male, 412 mm DW), same data as MZUSP 104247 View Materials . GoogleMaps
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
ParvPhylum |
Chondrichthyes |
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Potamotrygon scobina Garman, 1913
| João Pedro Fontenelle & Marcelo R. De Carvalho 2017 |
P. limai
| Fontenelle, Silva & Carvalho 2014 |
Potamotrygon scobina:
| Ferreira et al. 2011 |
Paratrygon scobina:
| Fowler 1948 |
Potamotrygon scobina
| Garman 1913 |